Question
Redox reactions are common in the chemistry of Group 17.
Which statement is correct?
A \(Br^–\) ions will reduce \(Cl_2\) but not \(I_2\).
B \(Cl_2\) will oxidise \(Br^–\) ions but not \(I^–\) ions.
C \(F_2\) is the weakest oxidising agent out of \(F_2\), \(Cl_2\), \(Br_2\) and \(I_2\).
D \(I^–\) ions are the weakest reducing agent out of \(F^–\), \(Cl^–\), \(Br^–\) and \(I^–\).
Answer/Explanation
Ans: A
Question
X, Y and Z represent different halogens. The table shows the results of nine experiments in which aqueous solutions of X2, Y2 and Z2 were separately added to separate aqueous solutions containing X–, Y–and Z–ions.
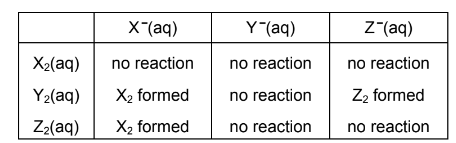
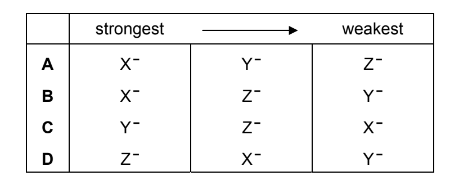
Which row of the following table contains the ions X–, Y– and Z– in order of their decreasing strength as reducing agents?
Answer/Explanation
Answer: B
Question
Astatine, At, is below iodine in Group VII of the Periodic Table.
Which statement is most likely to be correct?
A \( AgAt_{(s)}\) reacts with excess dilute aqueous ammonia to form a solution of a soluble complex.
B Astatine and KCl(aq) react to form KAt(aq) and chlorine.
C KAt(aq) and dilute sulfuric acid react to form white fumes of \(HAt_{(g)}\).
D \(NaAt_{(s)} \)and concentrated sulfuric acid react to form astatine.
Answer/Explanation
Ans:D
Question
On being heated, hydrogen iodide breaks down more quickly than hydrogen chloride. Which statements explain this faster rate?
1 The HI bond is weaker than the HCl bond.
2 The reaction of the breakdown of HI has a smaller activation energy than that of HCl.
3 The breakdown of HI is more exothermic than that of HCl.
The responses A to D should be selected on the basis of
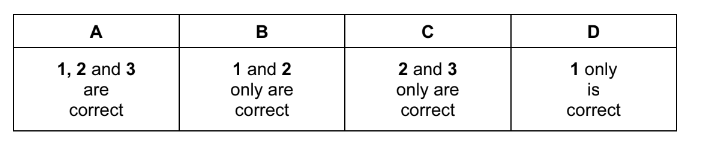
▶️Answer/Explanation
Ans:B
Question
Redox reactions occur very frequently in the chemistry of Group VII. Which statement is correct?
A Chlorine will oxidise bromide ions but not iodide ions.
B Fluorine is the weakest oxidising agent out of\( F_{2}, Cl_{2}, Br_{2}\) and\( I_{2}\).
C Iodide ions are the weakest reducing agent out of \(F^-, Cl^-, Br ^-\) and \(I^ -\).
D When chlorine reacts with water, chlorine is both oxidised and reduced.
Answer/Explanation
Ans:D
Question
When a red-hot platinum wire is plunged into a test tube of hydrogen iodide, the gas is decomposed into its elements. If the experiment is repeated with hydrogen chloride, no change occurs. Which factors contribute to this behaviour?
1 the strength of the hydrogen-halogen bond
2 the size of the halogen atom
3 the standard enthalpy of formation, \(\Delta H_f^\Theta \) , of each of the products of decomposition
The responses A to D should be selected on the basis of
Answer/Explanation
Ans: B
Question
Element 85, astatine, is in Group VII. Concentrated sulfuric acid is added to sodium astatide. The mixture of products includes astatine, hydrogen astatide, hydrogen sulfide, and sodium sulfate.
Which product is formed by the oxidation of one of the constituents of sodium astatide?
A astatine
B hydrogen astatide
C hydrogen sulfide
D sodium sulfate
Answer/Explanation
Ans: A