Question
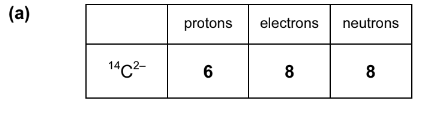
(a) Atoms and ions of elements are made up from the three subatomic particles, protons, electron and neutrons, in varying amounts. Complete the following table to show the number of each particle in \(^{14}C^{2-}\)

(b) Describe the observations you would make during the reactions, if any, of the following chlorides with water.
Write equations for any reactions that occur.
\(CCl _{4}\) observation
equation
\(GeCl_{ 4}\) observation
equation \(SnCl_{ 4}\) observation equation
(c) Suggest a reason for any difference in the reactivities of the chlorides given in (b).
(d) Use data from the Data Booklet to explain why an aqueous solution of \(SnCl _{2}\) reacts with\( Cl_{ 2}\)(g) but an aqueous solution of \(PbCl _{2}\) does not.
Write an equation for the reaction.
(e) (i) State the relationship between the Faraday constant and the Avogadro constant.
(ii) When a current of 1.2 A was passed through dilute sulfuric acid for 30 minutes, it was found that 130\( cm^{3}\) of oxygen, measured at 25 °C and 1 atm, was collected at the anode.The following reaction takes place.
\(2H_{2}O(l)\) → \(4H+(aq) + O_{2}(g) + 4e^{–}\)
Use these data and data from the Data Booklet to calculate a value for the Avogadro constant, L, by calculating
• the number of moles of oxygen produced,
• the number of moles of electrons needed for this,
• the number of coulombs passed,
• the number of electrons passed,
• the number of electrons in one mole of electrons (L).
Answer/Explanation
(b) \(CCl_{4}\): no reaction
\(GeCl_{4} \)and \(SnCl_{4}\): for each steamy fumes evolved or white solid produced
\(GeCl_{4} + 2H_{2}O \)→ \(GeO_{2} + 4HCl\)
\(SnCl_{4} + 2H_{2}O\) → \(SnO_{2} + 4HCl\)
(c) Ge/Sn use d–orbitals
or Ge /Sn have low lying d orbitals
or carbon cannot expand its octet
or carbon cannot accommodate more than 4 bonded pairs
(d) \( Sn^{4+} /Sn^{2+}\) = +0.15V and \(Pb^{4+} /Pb^{2+}\) = +1.69V and\( Cl_{2} /Cl^{–}\) = + 1.36V
\(Sn^{2+}\) is oxidised by \(Cl_{2}\) because its \(E^{\Theta}\) is less positive/more negative or\( Sn^{2+}\) is a good reducing agent due to its smaller E value than \(Cl_{2}\) ora
or\( Pb^{4+}\) is a stronger oxidising agent than \(Cl_{2}\) so \(Pb^{2+} \)with\( Cl^{2}\) reaction is not feasible or\( Sn^{4+}\) is a weaker oxidising agent than \(Cl_{2}\) so \(Sn_{2+}\) with\( Cl_{2}\) reaction is feasible
\(SnCl_{2} + Cl_{2}\) → \(SnCl_{4}\)
or\( Sn_{2}+ + Cl_{2}\) → \(Sn^{4+}\) + 2Cl^{–}\) or \(SnCl_{2} + Cl_{2} + 2H_{2}O\) → \(SnO_{2} + 4HCl\)
(e) (i) F = Le
(ii) moles of O2(g) = 130/24000 = \(5.417 x 10^{–3}\) mol
moles of electrons needed = 4 x 5.417 x 10–3 or 2.17 x \(10^{–2}\) mol
no. of coulombs passed = 1.2 x 30 x 60 or 2160 C
no. of electrons passed = 2160/ 1.6 x 10–19 or 1.35 x 1022
no. of electrons per mole = \(1.35 x 10^{22} / 2.17 x 10^{–2}\) =\( 6.2 x 10^{23} (mol^{–1})\)
Question
(a) In this question, K, L and M refer to a halogen atom or halide ion. For each part question, read the information and complete the answer lines below.
(i) When concentrated sulfuric acid is added to solid NaK, white fumes are produced that turn damp blue litmus paper red. No other colour changes are observed. identity of K = equation for reaction
explanation of observation
(ii) When silver nitrate solution is added to an aqueous solution of NaL, a precipitate forms that remains after the addition of concentrated ammonia solution. identity of L = colour of precipitate equation for reaction
(iii) M2 is a liquid at room temperature with a boiling point higher than that of chlorine but lower than that of iodine. identity of M =
explanation
(b) The diagram below is a simplified representation of a diaphragm cell.
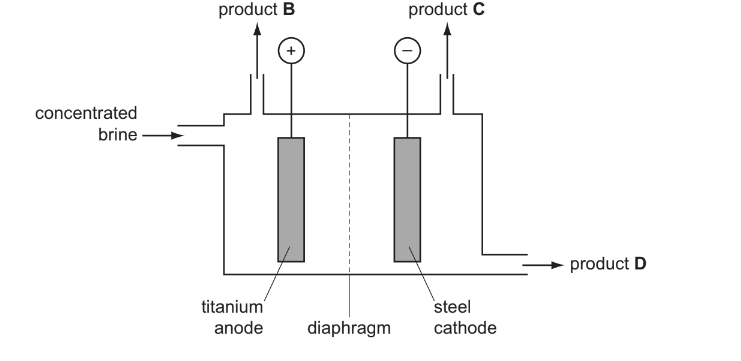
(i) Identify each of the products. B , C , D
(ii) Give the equations for the two electrode reactions.
anode
cathode
▶️Answer/Explanation
(a) (i) $\mathbf{K}=\mathrm{C} \tau /$ chloride $/ \mathrm{F}^{-} /$fluoride
$\mathrm{H}_2 \mathrm{SO}_4+2 \mathrm{NaC} l \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{HC} l$ (or equation with $\mathrm{F}$ or $\mathbf{K}$ for $\mathrm{Cl}$ ) OR $\mathrm{H}_2 \mathrm{SO}_4+\mathrm{NaC} l \rightarrow \mathrm{NaHSO}_4+\mathrm{HC} l$ (or equation with $\mathrm{F}$ or $\mathbf{K}$ for $\mathrm{C} l$ )
ecf from identity of $\mathbf{K}$ so long as halide
$\mathrm{HK}$ is acidic/HK is a gas/an acidic gas is produced
(ii)
L =\( I^{–} / iodide\)
colour = yellow ecf from identity of L i.e.\( Cl^{–}(white) or Br^{–}\)(cream)
\(Ag^{+}+I^{-}\rightarrow AgI\) (or equation with L)
\(AgNO_{3}+NaI\rightarrow AgI+NaNO_{3}\) (or equation with L) ecf from identity of L so long as halide
(iii) $\quad \mathrm{Br}_2 /$ bromine has fewer electrons than iodine/more electrons than chlorine intermolecular/van der Waals’ forces (in $\mathrm{Br}_2 / \mathbf{M}_2$ ) weaker than in iodine/stronger than in chlorine
(b) (i)
B =\( chlorine/Cl_{ 2}\)
C = \(hydrogen/H_{2}\)
D = sodium hydroxide/NaOH
(ii) anode: 2Cl^{–} \rightarrow
cathode:\( 2H^{+} + 2e^{–} \rightarrow H_{2}\) OR
\(2H_{2}O + 2e^{–} \rightarrow 2OH^{–} + H_{2}\)