Question
The first stage in the chloride process for the manufacture of titanium consists of the following reaction.
$
2 \mathrm{TiO}_2+4 \mathrm{Cl}_2+3 \mathrm{C} \rightarrow 2 \mathrm{TiCl}_4+2 \mathrm{CO}+\mathrm{CO}_2
$
What is reduced in this reaction?
A carbon
B chlorine
C oxygen
D titanium
▶️Answer/Explanation
Ans:B
Question
Chlorine reacts with sodium hydroxide in two different ways depending upon the temperature.
reaction 1 \(~~~C\imath_{2}~+~2OH\rightarrow C \imath^{-}~+~C \imath O^{-} ~+~H_{2}O\)
reaction 2 \(~~~3C \imath_{2}~+~6OH^{-}\rightarrow 5C \imath ~+~C \imath O_{3}^{~-}~+~3H_{2}O\)
Which statements about these reactions are correct?
1 Reaction 2 requires a higher temperature than reaction 1.
2 The products of reaction 1 show chlorine in two different oxidation states.
3 The products of reaction 2 show oxygen in two different oxidation states.
▶️Answer/Explanation
Ans:B
Question
What is the order of increasing melting point of the three chlorides shown?
\(CC \imath_{4}~~~~~MgCl_{2}~~~~~PC \imath_{5}\)
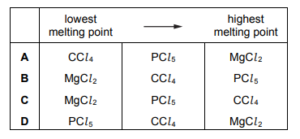
▶️Answer/Explanation
Ans:A
Question:
Hot aqueous sodium hydroxide reacts with chlorine.
\(6NaOH(aq)~+~3C\imath_{2}(g)\rightarrow 5NaC \imath~+~NaC \imath O_{3}(aq)~+~3H2O(l)\)
Which statement is correct?
A The oxidation numbers of chlorine and hydrogen both change in the reaction.
B The oxidation numbers of chlorine in the products are –1 and +1.
C If the aqueous sodium hydroxide is cold the reaction produces NaClO instead of NaClO3.
D Sodium undergoes disproportionation in this reaction.
▶️Answer/Explanation
Ans:C
Question
Three test-tubes, $X, Y$, and $Z$, each contain a small amount of water.
- A small amount of $\mathrm{NaC} l$ is added to test-tube $\mathrm{X}$.
- A small amount of $\mathrm{SiCl}_4$ is added to test-tube $\mathrm{Y}$.
- A small amount of $\mathrm{A} l \mathrm{C} l_3$ is added to test-tube $Z$.
After a short time, two drops of Universal Indicator solution are added to each test-tube.
Which observations are made?
1 The indicator added to test-tube $X$ stays green.
2 The indicator added to test-tube $Y$ turns red.
3 The indicator added to test-tube $Z$ turns red.
▶️Answer/Explanation
Ans:A
Question
Chlorine atoms in the upper atmosphere cause the breakdown of ozone.
$
\begin{aligned}
& \mathrm{Cl}+\mathrm{O}_3 \rightarrow \mathrm{O}_2+\mathrm{ClO} \\
& \mathrm{ClO}+\mathrm{O} \rightarrow \mathrm{Cl}+\mathrm{O}_2
\end{aligned}
$
Which statements are correct when referring to these chlorine atoms?
1 The chlorine atoms act as catalysts.
2 The chlorine atoms are free radicals.
3 The chlorine atoms are formed by heterolytic fission of a covalent bond in chlorofluorocarbons.
▶️Answer/Explanation
Ans:B
Question
Chlorine gas is widely used to treat contaminated water.
Which species present in water when chlorine gas has been added is responsible for killing bacteria?
A $\mathrm{ClO}_2^{-}$
B $\mathrm{Cl}^{-}$
C $\mathrm{HCl}$
D $\mathrm{OCl}^{-}$
▶️Answer/Explanation
Ans:D
Question
The presence of a halogen in an organic compound may be detected by warming the organic
compound with aqueous silver nitrate.
Which compound would be the quickest to produce a precipitate?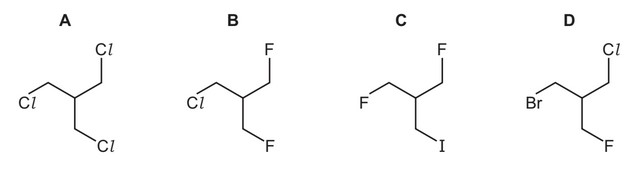
Answer/Explanation
Ans: C
Question
The results of tests performed on a white crystalline solid, X, are given in the table.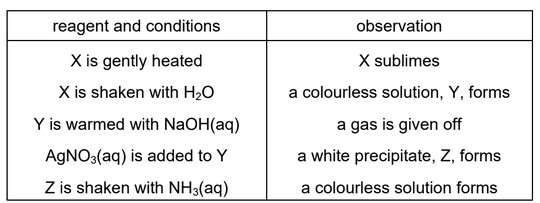
What is the identity of X?
A aluminium bromide
B aluminium chloride
C ammonium bromide
D ammonium chloride
Answer/Explanation
Ans: D
Question
Silicon is heated in an excess of chlorine, producing compound J.
An excess of water is added to the sample of J produced.
Which row is correct?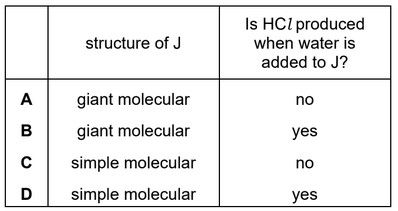
Answer/Explanation
Ans: D
Question
Chlorine atoms in the upper atmosphere cause the breakdown of ozone.
Cl + O3 → O2 + ClO
ClO + O → Cl + O2
Which statements about these chlorine atoms are correct?
1 The chlorine atoms act as catalysts.
2 The chlorine atoms are free radicals.
3 The chlorine atoms are formed by heterolytic fission of a covalent bond in chlorofluorocarbons.
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
B
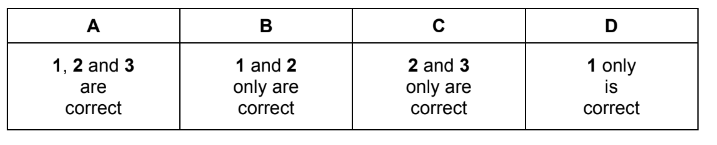
Question
Which statements are correct?
1 Chloride ions are oxidised when concentrated sulfuric acid is added to NaCl(s).
2 A disproportionation reaction takes place when chlorine is added to cold NaOH(aq).
3 An acidic solution forms when chlorine is added to water.
▶️Answer/Explanation
Answer C
Question
Which substance reacts with trichloroethene to give a chiral product?
A Br2 B HCl C NaCN D NaOH
Answer/Explanation
Answer A
Question
Chlorine and bromine have different volatilities.
Which row identifies the more volatile of the two elements, and gives the correct explanation?
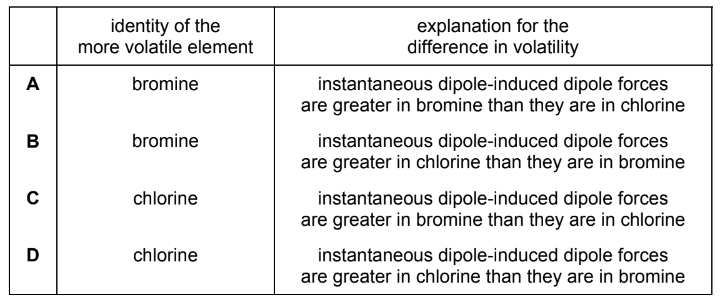
Answer/Explanation
Answer C
Question
In the chemical equation, w, x, y and z are all whole numbers.
\(wClO_{3}^{-} + xMnO_{4}^{-} + yH^{+} \rightarrow wClO_{4}^{-} + xMnO_{2} + zH_{2}O\)
When the equation is balanced, what are w, x and y?
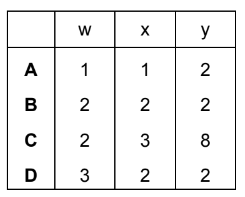
Answer/Explanation
Answer D
Question
Reaction 1: chlorine reacts with cold aqueous sodium hydroxide to form solution Z.
Reaction 2: solution Z is heated and forms ClO3–(aq) and Cl– (aq).
Which equations represent reaction 1 and reaction 2?
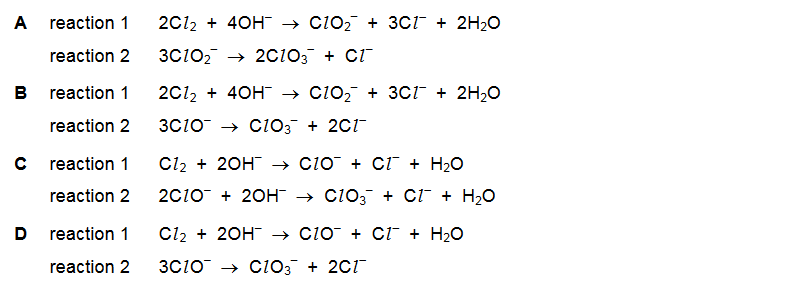
Answer/Explanation
Answer D
Question
Chlorine reacts with cold aqueous sodium hydroxide to produce sodium chloride, water and compound X.
Chlorine reacts with hot aqueous sodium hydroxide to produce sodium chloride, water and compound Y.
What are the oxidation states of chlorine in compound X and compound Y?
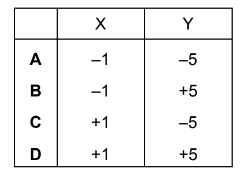
Answer/Explanation
Answer D
Question
A solution of sodium hydroxide reacts with 3 mol of chlorine under certain conditions. The reaction
produces 5mol of sodium chloride and 1 mol of X, the only other chlorine-containing product.
What is the formula of compound X?
A NaClO B \(NaClO_2\) C \(NaClO_3\) D \(NaClO_4\)
Answer/Explanation
Ans: C
Question
The first propagation step in the reaction between methane and chlorine is shown.
CH4 + Cl • → CH3• + HCl
How many different first propagation steps are possible in the reaction between pentane and chlorine?
A 2 B 3 C 4 D 5
Answer/Explanation
Answer: B
Question
Gaseous phosphorus pentachloride can be decomposed into gaseous phosphorus trichloride and chlorine by heating. The table gives the bond energies.

What is the enthalpy change for the decomposition of PCl5 to PCl3 and Cl2?
A –418kJ mol–1
B –88kJ mol–1
C +88kJ mol–1
D +418kJ mol–1
Answer/Explanation
Answer: D
Question
Chlorine is widely used in water treatment plants.
Which reaction takes place when chlorine dissolves in water?
A \( Cl 2 + H_{2}O → HCl + HClO\)
B \(2Cl _{2} + 2H_{2}O → 3HCl + HClO_{2}\)
C \( 3Cl _{2} + 3H_{2}O → 5HCl + HClO_{3}\)
D \( 4Cl _{2} + 4H_{2}O → 7HCl + HClO_{4}\)
Answer/Explanation
Ans:A
Question
In the industrial electrolysis of brine to manufacture chlorine, the diaphragm used is a porous screen which allows the flow of electrolytes but keeps other chemicals separate. Which substance needs to be kept separate from the chlorine by the diaphragm?
A hydrogen
B sodium chloride
C sodium hydroxide
D water
Answer/Explanation
Ans:C
Question
The addition of aqueous silver nitrate to aqueous barium chloride produces a white precipitate which dissolves in excess dilute aqueous ammonia to form a colourless solution. The addition of excess dilute nitric acid to the colourless solution produces a white precipitate, Z.
What is Z?
A AgCl B \(BaCl _{2}\) C \(Ba(NO_{3})_{2}\) D \(NH_{4}NO_{3}\)
Answer/Explanation
Ans:A
Question
The table shows the physical properties of four substances. Which substance could be hydrogen chloride?
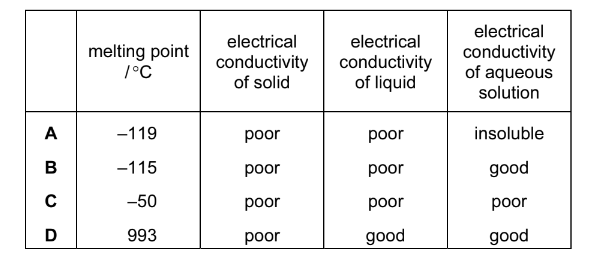
Answer/Explanation
Ans:B
Question
What is the order of increasing melting point of the four chlorides shown?
\(CCl_{ 4} \) HCl \( MgCl _{2}\) \( PCl _{5}\)
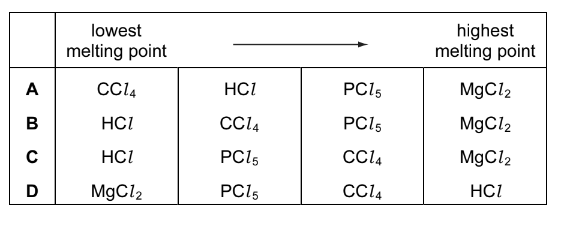
Answer/Explanation
Ans:B
Question
Which molecules would be present in the mixture produced by the photochemical chlorination of methane?
1 hydrogen
2 hydrogen chloride
3 dichloromethane
The responses A to D should be selected on the basis of
Answer/Explanation
Ans: C
Question
Which reagents and conditions will convert propane into 1-chloropropane?
- Cl2 and sunlight
- conc. HCl, reflux
- PCl5
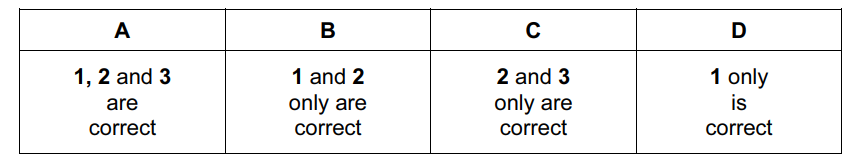
Answer/Explanation
Ans:
D
Question
High-energy radiation in the stratosphere produces free-radicals from chlorofluoroalkanes, commonly known as CFCs.
Which free-radical is most likely to result from the irradiation of CHFClCF2Cl?
- \(CHFCl \dot{C}FCl \)
- \(\dot{C}HCl F_{2}Cl\)
- \(\dot{C}HFCF_{2}Cl\)
- \(\dot{C}FCl CF_{2}Cl\)
Answer/Explanation
Ans:
C
Question
Elements X and Y are both in period three.
When the chloride of X is added to water, it reacts and a solution of pH 2 is produced.
When the chloride of Y is added to water, it dissolves and a solution of pH 7 is produced.
Which statement explains these observations?
- Both chlorides hydrolyse in water.
- X is phosphorus and Y is aluminium.
- X is silicon and Y is sodium.
- X is sodium and Y is phosphorus.
Answer/Explanation
Ans:
C
Question
The depletion of the ozone layer in the upper atmosphere reduces the Earth’s natural protection from harmful ultraviolet radiation.
Which compound would cause the most depletion of the ozone layer?
A CCl3F B CF4 C CHClF2 D CH2F2
Answer/Explanation
Ans:
A