Question:
\(NO,~NO_{2}~CO\) and unburnt hydrocarbons are present in the exhaust gases of internal combustion engines. When catalytic converters are used to remove these compounds from the exhaust
gases, redox reactions occur. What happens to each compound in the catalytic converter?
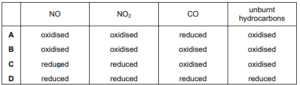
▶️Answer/Explanation
Ans:C
Question:
When concentrated sulfuric acid reacts with sodium iodide the products include sulfur, iodine, hydrogen sulfide and sulfur dioxide. Which statement is correct?
A Hydrogen sulfide is the product of a reduction reaction.
B Iodide ions are stronger oxidising agents than sulfate ions.
C Sulfur atoms from the sulfuric acid are both oxidised and reduced.
D Sulfur atoms from the sulfuric acid are oxidised to make sulfur dioxide
▶️Answer/Explanation
Ans:A
Question:
Nitrogen dioxide gas is produced when petrol is burned in car engines. Which acids are made in the atmosphere as a result of this release of nitrogen dioxide into the air?
1 \(H_{2}SO_{3}\)
2 \(H_{2}SO_{4}\)
3 \(HNO_{3}\)
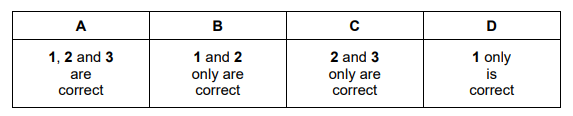
▶️Answer/Explanation
Ans:C
Question
Sulfur dioxide, $\mathrm{SO}_2$, reacts with calcium hydroxide in aqueous solution.
What is the main product that is first formed?
A $\mathrm{Ca}\left(\mathrm{HSO}_4\right)_2$
B CaS
C $\mathrm{CaSO}_3$
D $\mathrm{CaSO}_4$
▶️Answer/Explanation
Ans:C
Question:
Carbon and nitrogen are adjacent in the Periodic Table.
Which properties do they both have?
1 There is an empty 2p orbital in each atom of the element.
2 The principal quantum number of the highest occupied orbital is 2.
3 They can form compounds in which their atoms form four bonds.

▶️Answer/Explanation
Ans:C
Question:
Which statement about nitrogen or its compounds is correct?
A In the Haber process the temperature is kept high to give a good equilibrium yield of
ammonia.
B Nitrogen gas is unreactive because of the strong nitrogen–nitrogen double bond.
C Nitrogen monoxide will react with carbon monoxide under suitable conditions.
D The formula of ammonium sulfate is \(NH_{4}SO_{4}\).
▶️Answer/Explanation
Ans:C
Question
Which reagent, when mixed with ammonium sulfate and then heated, liberates ammonia?
A aqueous bromine
B dilute hydrochloric acid
C limewater
D potassium dichromate(VI) in acidic solution
▶️Answer/Explanation
Ans:C
Question
In some rice-growing parts of the world, farmers use a combination of paddy fields and a fish farm. Rice paddy fields are flooded for much of the growing cycle and water running off the fields flows through pens where fish are raised. Nitrogen-based fertilisers are generally very soluble in water.
Which problems could result from farmers applying excess nitrogen-based fertilisers to their paddy fields?
1 decreased fish production in the fish pens
2 decreased levels of oxygen in the water
3 increased growth of algae in the fish pens
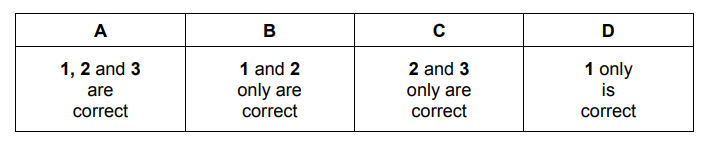
▶️Answer/Explanation
Ans:A
Question
Ammonium sulfate, $\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4$, and ammonium nitrate, $\mathrm{NH}_4 \mathrm{NO}_3$, are used as fertilisers.
These salts have different percentages by mass of nitrogen. They have the same effect as each other on the $\mathrm{pH}$ of neutral soil.
Which row is correct?
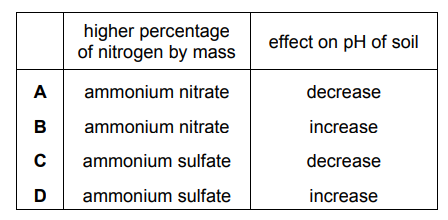
▶️Answer/Explanation
Ans:B
Question
Carbon monoxide, CO, nitrogen dioxide, \(NO_2\), and sulfur dioxide, \(SO_2\), are all atmospheric pollutants.
Which reaction occurs in the atmosphere?
A CO is spontaneously oxidised to \(CO_2\).
B \(NO_2\) is reduced to NO by \(SO_2\).
C \(NO_2\) is reduced to NO by CO.
D \(SO_2\) is oxidised to \(SO_3\) by \(CO_2\).
Answer/Explanation
Ans: B
Question
Acid rain is a dilute solution of sulfuric acid.
Which pollutant also contributes to the formation of acid rain?
A carbon monoxide
B carbon dioxide
C nitrogen dioxide
D hydrocarbons
Answer/Explanation
Answer: C
Question
Ammonia, NH3, and hydrazine, NH2NH2, are two compounds of nitrogen, N2.
Which statement is correct?
A The N–N bond in NH2NH2 is polar.
B NH3 and NH2NH2 have lone pairs of electrons but N2 does not.
C The oxidation number of each nitrogen in NH2NH2 is +2.
D The reaction of nitrogen with hydrogen has a high activation energy.
Answer/Explanation
Answer:
D
Question
Nitrogen gas is unreactive, whereas oxygen gas and chlorine gas are reactive.
Which statements help to explain this difference?
1 The two N atoms in an N2 molecule are held together by a very strong triple bond.
2 The triple bond between two N atoms is not polar. The bonds in O2 and Cl2 are polar.
3 The atoms in N2 have a full outer shell of electrons. The atoms in O2 and Cl2 do not have a
full outer shell of electrons.
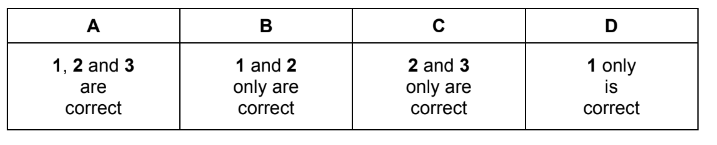
▶️Answer/Explanation
Answer D
Question
Nitric acid is known to take part in the oxidation of atmospheric sulfur dioxide. One possible reaction is shown.
\(SO_{2} + HNO_{3} \rightarrow NO^{+} + HSO_{4}^{-}\)
Which row shows the correct changes in oxidation numbers of nitrogen and sulfur?
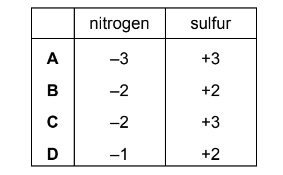
Answer/Explanation
Answer B
Question
Which statements correctly describe an oxide of nitrogen acting as an atmospheric pollutant?
1 Nitrogen monoxide, NO, reacts with oxygen to form nitrogen dioxide which contributes to acid rain.
2 Nitrogen dioxide reacts with sulfur dioxide to form sulfur trioxide which reacts with water to form sulfuric acid.
3 Nitrogen oxides react with unburnt hydrocarbons in sunlight to form other pollutants.
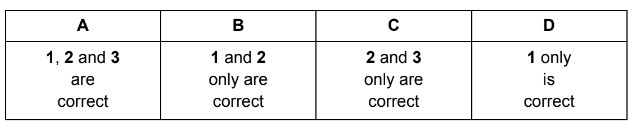
Answer/Explanation
Answer A
Question
Oxides of nitrogen are present in the environment due to natural and man-made sources.
Which row is correct?

Answer/Explanation
Answer A
Question
In which different forms does nitrogen exist in compounds?
1 bonded by a triple covalent bond
2 as part of a cation
3 in an oxidation state of +5
The responses A to D should be selected on the basis of
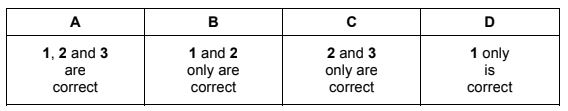
Answer/Explanation
Answer:
A
Question
When burned, sulfur forms a gaseous product X which can be oxidised to produce a gas Y. Gas Y reacts with water to produce a product Z.
Which row correctly shows the oxidation states of sulfur in X, Y and Z?
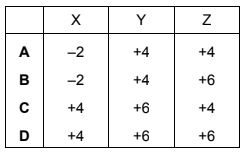
Answer/Explanation
Answer:
D
Question
Which statements explain why nitrogen gas is unreactive?
1 Nitrogen atoms are highly electronegative.
2 Nitrogen molecules are non-polar.
3 The triple bond between nitrogen atoms is very strong.
The responses A to D should be selected on the basis of
Answer/Explanation
Ans: C
Question
Which fertiliser contains the greatest percentage of nitrogen by mass?
A ammonium nitrate, \(NH_4NO_3\)
B ammonium sulfate, \((NH_4)_2SO_4\)
C diammonium hydrogen phosphate, \((NH_4)_2HPO_4\)
D urea, \(CO(NH_2)_2\)
Answer/Explanation
Ans: D
Question
Which statement about nitrogen or its compounds is correct?
A In the Haber process the temperature is kept high to give a good equilibrium yield of ammonia.
B Nitrogen gas is unreactive because of the strong nitrogen-nitrogen double bond.
C Nitrogen monoxide will react with carbon monoxide under suitable conditions.
D The formula of ammonium sulfate is \(NH_4SO_4\).
Answer/Explanation
Ans: C
Question
Which reaction does not contribute to the problem of acid rain?
A the combustion of fossil fuels
B the oxidation of sulfur dioxide to sulfur trioxide catalysed by nitrogen dioxide
C the reaction between nitrogen monoxide and carbon monoxide in a catalytic converter
D the reaction of sulfur trioxide with water
Answer/Explanation
Answer: C
Question
Which statements about the industrial manufacture of sulfuric acid are correct?
1 Sulfur is burned to form sulfur dioxide.
2 The stage that forms sulfur trioxide involves a \(V_{2}O_{5}\) catalyst.
3 The stage that forms sulfur trioxide is non-reversible.
The responses A to D should be selected on the basis of
Answer/Explanation
Ans:B
Question
Sulfur dioxide is used as a food preservative.
Which statements about sulfur dioxide, \(SO_{2}\), are correct?
1 \(SO_{2}\) behaves as an antioxidant.
2 Aqueous \(SO_{2}\) contains\( SO_{3}^{2–}\) ions.
3 \( SO_{2}\) inhibits the growth of mould and yeasts.
The responses A to D should be selected on the basis of
Answer/Explanation
Ans:A
Question
Total removal of the pollutant sulfur dioxide, is difficult. The quantities emitted from furnace chimneys can be lowered by using desulfurisation plants. The gases are reacted with calcium hydroxide to remove the \(SO_{2}\). What is the main product formed initially?
A \(Ca(HSO_{4})_{2} \) B CaS C \(CaSO_{3}\) D CaSO4
Answer/Explanation
Ans:C
Question
Use of the Data Booklet is relevant to this question.
When the liquid \(N_{2}F_{4}\) is heated, it decomposes into a single product, X. Which statements are correct?
1 N–F bonds are broken during this decomposition.
2 The enthalpy change when \(N_{2}F_{4}\) decomposes into X is approximately +160kJ \(mol^{1}\).
3 Molecules of X are non-linear.
The responses A to D should be selected on the basis of

Answer/Explanation
Ans:C
Question
The Contact process is used in the manufacture of sulfuric acid. The equation for the main reaction is shown below.
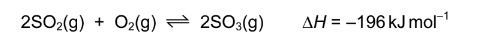
Which statement about this reaction is incorrect?
A Increased pressure gives a higher yield of\( SO)^{3}\).
B Increased temperature gives a higher yield of \(SO^{3}\).
C In the forward reaction the oxidation state of sulfur changes from +4 to +6.
D Vanadium(V) oxide is used as a catalyst.
Answer/Explanation
Ans:B
Question
Which reagent, when mixed and heated with ammonium sulfate, liberates ammonia?
A aqueous bromine
B dilute hydrochloric acid
C limewater
D potassium dichromate(VI) in acidic solution
Answer/Explanation
Ans: C
Question
Sulfur dioxide and sulfites are used in food preservation. Why are they used for this purpose?
1 They are reducing agents which slow down the oxidation of food.
2 They inhibit the growth of aerobic bacteria.
3 They react with \(NO_2(g)\) converting it to NO(g).
Answer/Explanation
Ans: B
Question
A metal, X, reacts with water to produce a colourless solution which gives a white precipitate
when mixed with aqueous sulfuric acid.
What is metal X?
A barium
B magnesium
C potassium
D sodium
Answer/Explanation
Ans: A
Question
How may nitrogen exist in compounds?
- bonded by a triple covalent bond
- as part of a cation
- having lost 3 electrons to form an anion
Answer/Explanation
Ans:
B
Question
In a car engine, non-metallic element X forms a pollutant oxide Y. Y can be further oxidised to Z. Two students made the following statements.
Student P The molecule of Y contains lone pairs of electrons.
Student Q The oxidation number of X increases by 1 from Y to Z.
X could be carbon or nitrogen or sulfur.
Which student could be correct if X were any of these elements?
- P only
- Q only
- both P and Q
- neither P nor Q
Answer/Explanation
Ans:
A
Question
Use of the Data Booklet is relevant to this question.
The nitrates of beryllium, calcium, magnesium, and strontium all decompose in the same way when heated. When 2.00 g of one of these anhydrous nitrates is decomposed, 1.32 g of gas is produced.
What is the nitrate?
- beryllium nitrate
- calcium nitrate
- magnesium nitrate
- strontium nitrate
Answer/Explanation
Ans:
B
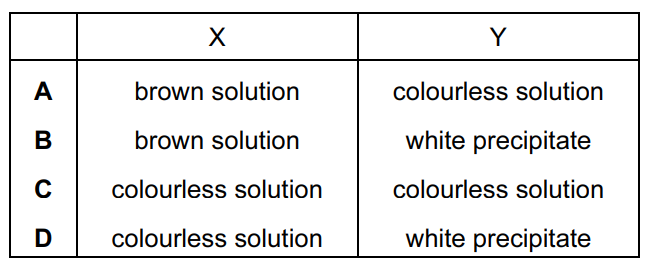
Question
The following two experiments are carried out with anhydrous potassium chloride and observations X and Y are made at the end of each experiment.
Concentrated sulfuric acid is added to the potassium chloride and the fumes produced are bubbled into aqueous potassium iodide solution – observation X.
The potassium chloride is dissolved in aqueous ammonia and this is then added to aqueous silver nitrate – observation Y.
What are the observations X and Y?
Answer/Explanation
Ans:
C