Question
(a) State one natural and one man-made occurrence of oxides of nitrogen.
(b) Under conditions of high pressure and a catalyst, nitrogen monoxide, NO, forms two other oxides of nitrogen, dinitrogen monoxide, N2O, and dinitrogen trioxide, N2O3.
…… NO(g) …… N2O(g) + …… N2O3(g) ΔHΘ= –195.2kJmol–1
ΔGΘ= –102.8kJmol–1
(i) Balance the equation above for the formation of N2O and N2O3 from NO.
(ii) State how the oxidation number of nitrogen changes during this reaction.
NO → N2O from ________________________ to ________________________
NO → N2O3 from ________________________ to ________________________
(iii) Calculate the entropy change for the reaction at 298K. Include the units in your answer.
(iv) State whether the sign of ΔSΘ calculated in (iii) agrees with that predicted from your balanced equation in (i). Explain your answer.
(c) At room temperature N2O3 dissociates.
N2O3(g) NO(g) + NO2(g)
(i) Write the expression for Kp for this equilibrium. Include the units in your answer.
A 1.00dm3 flask at 25°C is filled with pure N2O3(g) at an initial pressure of 0.60atm. At equilibrium, the partial pressure of NO2(g) is 0.48atm.
(ii) Calculate the partial pressures of NO(g) and N2O3(g) at equilibrium. Hence calculate the
value of Kp at 25°C.
(d) NO reacts readily with oxygen.
2NO(g) + O2(g) → 2NO2(g)
The table shows how the initial rate of this reaction at 25°C depends on the initial concentrations of the reactants.
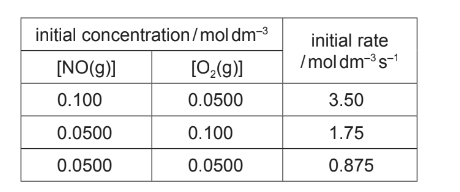
(i) Deduce the order of reaction with respect to each reactant. Explain your reasoning.
order with respect to [NO(g)] ________________________________
order with respect to [O2(g)] _________________________________
(ii) State the rate equation for this reaction. Use the rate equation to calculate the rate constant.
Include the units for the rate constant in your answer.
(e) NO reacts with iron pentacarbonyl, Fe(CO)5, as shown. NO and CO are both monodentate ligands.
Fe(CO)5 + 2NO → Fe(CO)2(NO)2 + 3CO
During this reaction the co-ordination number of the iron changes.
(i) State what is meant by the term co-ordination number.
(ii) Describe how the co-ordination number of the iron changes during this reaction.
from ______________________ to _____________________
(iii) Only one stereoisomer of Fe(CO)2(NO)2 exists.
Use this information to suggest the geometry of the complex.
(f) The complex Ru(NO)L2Cl3 exists in three isomeric forms. L represents the monodentate ligand C6H5P(CH3)2.
(i) Complete the three-dimensional diagrams to show the three isomers of Ru(NO)L2Cl3.
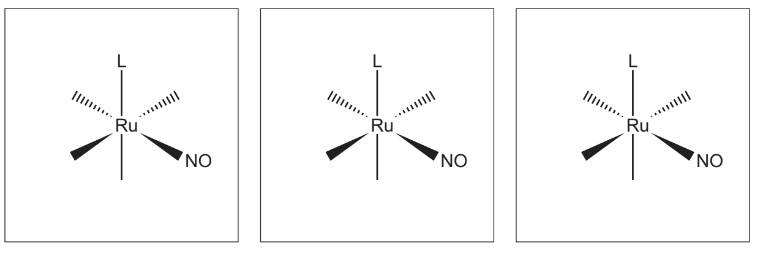
(ii) Suggest the type of isomerism shown.
Answer/Explanation
Answer: (a) natural: lightning, bacterial decomposition, volcanic emissions
man-made: exhaust fumes, power stations, jet / car/ vehicle engines
(b)(i) 4NO → N2O + N2O3
(b)(ii) +2 to +1 AND +2 to +3
(b)(iii) ∆S = (∆H –∆G) / T
= (–195.2+102.8) / 298
= –0.310 kJ mol–1K–1
M1 numerical answer
M2 units
(b)(iv) yes as there is a decrease in no. of moles of gas
OR yes as moles of (gaseous) reactants is greater than moles of (gaseous) products
(c)(i) Kp = p(NO)p(NO2) / p(N2O3)
AND units: atm OR Pa
(c)(ii) M1
p(NO) = p(NO2) = 0.48 atm
p(N2O3)eqm = p(N2O3)o – 0.48 = 0.12 atm
M2
Kp = 0. 482 / 0.12 = 1.92 (atm)
(d)(i) M1 from 3rd and 1st rows as [NO] × 2 , rate increases × 4, so order = 2
M2 from 3rd and 2nd rows as [O2] × 2, rate also × 2, so order = 1
(d)(ii) rate =k[NO]2[O2]
k = rate / ([NO]2[O2]) = 3.5 / (0.01 × 0.05) = 7000 units: mol–2 dm6 s–1
(e)(i) the number of dative bonds formed with / by the central metal atom / ion
OR number of bonds between the ligands and the central metal atom / ion
(e)(ii) from 5 to 4
(e)(iii) tetrahedral
(f)(i)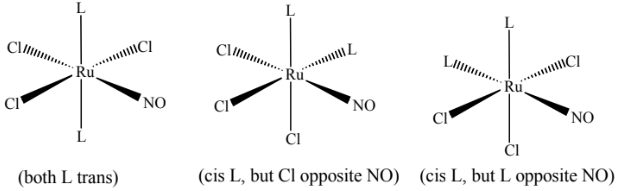
(f)(ii) geometric(al) OR cis-trans
Question
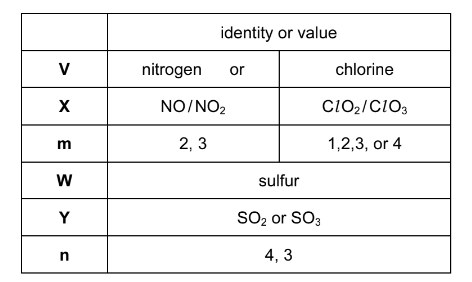
Two elements, V and W, are in adjacent groups in the Periodic Table.
V reacts with oxygen to form an acidic gas, X. V forms an anion with formula VOm–.
W reacts with oxygen to form an acidic gas, Y. W forms an anion with formula WOn2–.
A solution of WOn2– forms a white precipitate with Ba2+(aq) but shows no visible reaction with Mg2+(aq).
(a) Complete the table below.

(b) By referring to enthalpy changes, explain why WOn2– forms a white precipitate with Ba2+(aq) but shows no visible reaction with Mg2+.
Answer/Explanation
Answer: (a)
(b) M1: (white precipitate is BaSO4) descending the group ∆Hsol becomes more endothermic / positive;
M2, M3 any two from:
∆Hlatt decreases /becomes more endothermic / becomes less exothermic
∆Hhyd decreases / becomes more endothermic / becomes less exothermic
∆Hhyd decreases more than ∆Hlatt