Question
The skeletal formula of compound X is shown.
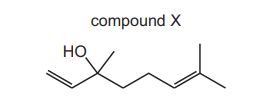
What is the molecular formula of compound X?
A $\mathrm{C}_{10} \mathrm{H}_{18} \mathrm{O}$
B $\mathrm{C}_{10} \mathrm{H}_{20} \mathrm{O}$
c $\mathrm{C}_{11} \mathrm{H}_{22} \mathrm{O}$
D $\mathrm{C}_{11} \mathrm{H}_{24} \mathrm{O}$
▶️Answer/Explanation
Ans:A
Question:
Which pair of compounds are functional group isomers of each other?
A butan-1-ol and butanal
B ethylpropanoate and pentanoic acid
C hex-1-ene and hex-2-ene
D propylamine and propanenitrile
▶️Answer/Explanation
Ans:B
Question
A gaseous hydrocarbon has a density of 2.42 g dm-3 under room conditions.
What could be the skeletal formula of this hydrocarbon?
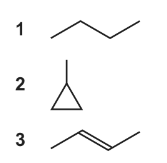
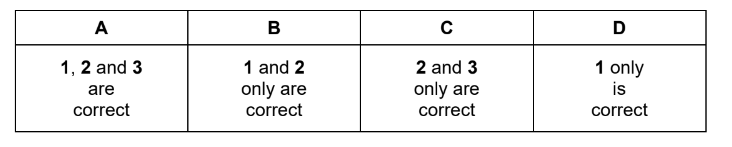
Answer/Explanation
Answer: D
Question
What is the structural formula of the major product when hydrogen bromide reacts with 2-methylbut-2-ene?
A CH2BrCH(CH3)CH2CH3
B (CH3)2CBrCH2CH3
C (CH3)2CHCHBrCH3
D (CH3)2CHCH2CH2Br
Answer/Explanation
Answer B
Question
Which compound has the molecular formula C6H10O?
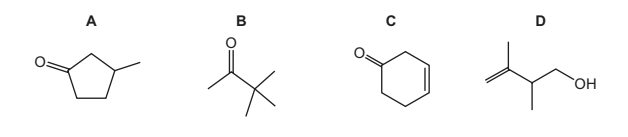
Answer/Explanation
Answer A
Question
Structural isomerism and stereoisomerism should be considered when answering this question. The molecular formula of compound X is C5H12O.
Compound X:
● reacts with alkaline aqueous iodine
● can be dehydrated to form two alkenes only.
What could be the identity of compound X?
A CH3CH2CH(CH3)CH2OH
B CH3CH2CH(OH)CH2CH3
C (CH3)2CHCH(OH)CH3
D CH3CH2CH2CH(OH)CH3
Answer/Explanation
Answer:
C
Question
What is the name of compound X?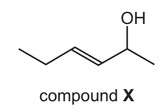
A trans-2-hydroxyhex-3-ene
B trans-2-hydroxyhexene
C trans-5-hydroxyhex-3-ene
D trans-5-hydroxyhexene
Answer/Explanation
Ans: A
Quesrion
The diagram shows the structure of propanamide.
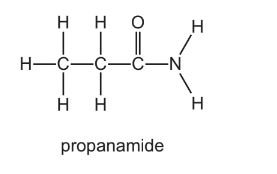
Which statements about the hydrolysis of propanamide are correct?
1 Propanamide can be hydrolysed by heating under reflux with \(H_{2}SO_{4}\)(aq).
2 Propanamide can be hydrolysed by heating under reflux with NaOH(aq).
3 Propanamide can be hydrolysed by cold water.
The responses A to D should be selected on the basis of
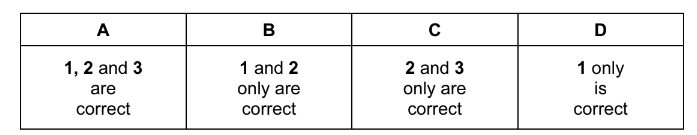
Answer/Explanation
Ans:B
Question
Hexamine is a crystalline solid used as a fuel in portable stoves. The diagram shows its skeletal structure.
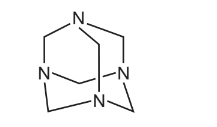
What is the empirical formula of hexamine?
A \( CH_{2}N \) B \(C_{3}H_{6}N_{2}\) C \(C_{4}H_{8}N_{4}\) D \(C_{6}H_{12}N_{4}\)
Answer/Explanation
Ans:B
Question
An organic compound J reacts with sodium to produce an organic ion with a charge of –3. J reacts with NaOH(aq) to produce an organic ion with a charge of –1.
What could be the structural formula of J?
- HO2CCH(OH)CH2CO2H
- HO2CCH(OH)CH2CHO
- HOCH2CH(OH)CH2CO2H
- HOCH2COCH2CHO
Answer/Explanation
Ans:
C