Question
The following compounds were all found to be components of a sample of petrol.
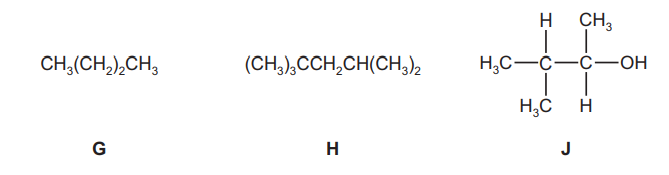
(a) (i) Give the molecular formula of compound G.
……………………………………………………………………………………………………………………… [1]
(ii) Give the empirical formula of compound H.
……………………………………………………………………………………………………………………… [1]
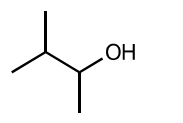
(iii) Draw the skeletal formula of compound J.[1]
(b) Write an equation to represent the complete combustion of compound H.
……………………………………………………………………………………………………………………………. [1]
(c) Fossil fuels are often contaminated with sulfur.
State and explain why supplies of fossil fuels that contain sulfur pose a problem to the environment.
(d) The boiling points of compounds G, H and J are shown below.
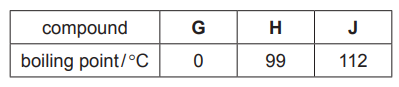
Explain the differences in the boiling points of the three compounds.
(e) Compound $\mathbf{J}$ can be produced from 2-chloro-3-methylbutane, $\mathrm{C}_5 \mathrm{H}_{11} \mathrm{Cl}$.
Give the reagent(s) and conditions for this reaction.[1] [Total: 11]
▶️Answer/Explanation
Ans:
(a) (i) $\underline{\mathrm{C}}_4 \underline{H}_{10}$
(ii) $\quad \underline{\mathrm{C}}_4 \mathrm{H}_9$
(iii)
(b) $\quad \mathrm{C}_8 \mathrm{H}_{18}+12 \frac{1}{2} \mathrm{O}_2 \rightarrow 8 \mathrm{CO}_2+9 \mathrm{H}_2 \mathrm{O}$
(c) sulfur dioxide would be produced on combustion
(which contributes to) acid rain
(d) M1 = H has more/ greater/ stronger van der Waals’/ intermolecular forces than G / ora
M2 = (because)
H has more electrons (thanG)
M3= J has hydrogen bonding (between molecules)
M4 = strong(er)/ great(er) forces require AND high/ more energy to overcome
(e) NaOH(aq)
Question
2-methylbut-1-ene reacts with acidified manganate(VII) ions, under specific conditions, to produce two organic compounds $\mathbf{X}$ and $\mathbf{Y}$.
$\mathbf{X}$ immediately reacts with the acidified manganate(VII) ions to form carbon dioxide and water. $\mathbf{Y}$ has the structural formula $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCH}_3$.
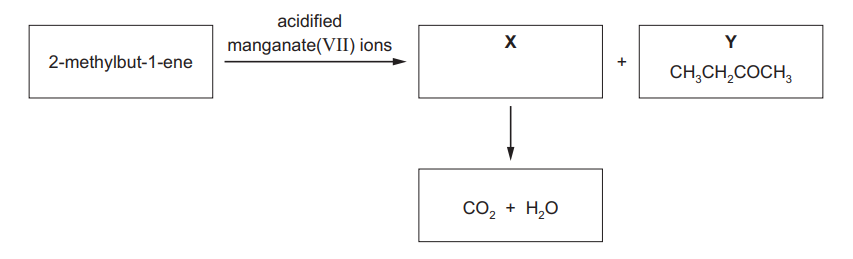
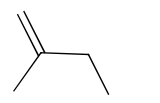
(a) Draw the skeletal formula of 2-methylbut-1-ene.[1]
(b) (i) State the specific conditions required for the acidified manganate(VII) ions to react with 2-methylbut-1-ene in this way.[1]
(ii) Name the type of reaction occurring to the functional group in 2-methylbut-1-ene in the reaction in $\mathbf{( b )}(\mathbf{( i )}$.[1]
(c) Draw the structural formula of $\mathbf{X}$.[1]
(d) Describe a chemical test and the expected observation(s) to confirm the presence of the carbonyl functional group in $\mathbf{Y}$.[5]
(e) The infra-red spectrum of 2-methylbut-1-ene is shown.
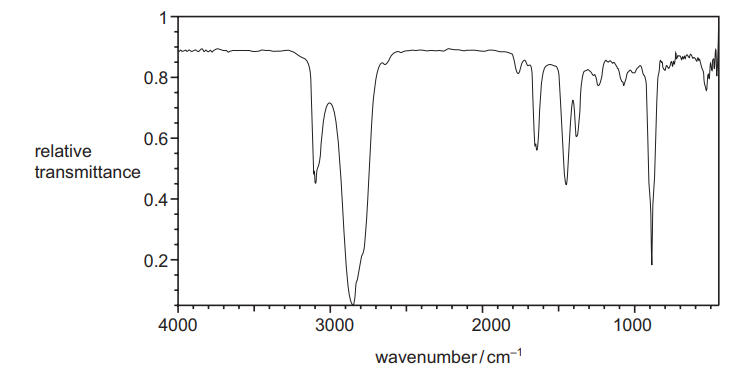
Predict two main differences that would be seen between the spectra of $\mathbf{Y}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCH}_3$, and of 2-methylbut-1-ene. Give reasons for your predictions.
Your answer should refer only to the region of each spectrum above $1500 \mathrm{~cm}^{-1}$.[2]
(f) Propanoic acid, $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$, is reduced by $\mathrm{LiAlH}_4$.
(i) Write an equation to show this reaction. Use $[\mathrm{H}]$ to represent an atom of hydrogen from the reducing agent.[1]
(ii) Name the organic product formed in this reaction.[1]
(g) Organic compound W is an ester which is a structural isomer of propanoic acid.
(i) State the molecular formula of W. [1]
(ii) Draw a possible structure of W.
▶️Answer/Explanation
Ans:
6(a)
6(b)(i) hot AND concentrated 1
6(b)(ii) oxidation
6(c)
Structural formula of $\boldsymbol{X}$ : $\mathrm{HCO}_2 \mathrm{H}$ OR HCOOH
6(d) M1 reagent (2,4–) DNPH / (2,4)-dinitrophenylhydrazine
M2 observation yellow / orange / red precipitate
6(e)
Predict two differences, with reasons, between spectra of $\mathbf{Y}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCH}_3$ and 2-methylbut-1-ene (shown)
first difference
M1 absence of peak/ absorption at $3100\left(\mathrm{~cm}^{-1}\right)$ as no longer any $=\mathrm{C}-\mathrm{H}$ present (in $\mathbf{Y}$ )
second difference
M2 peak at $1650\left(\mathrm{~cm}^{-1}\right)$ moves to the left to any value / range of values between 1670 and 1740) due to disappearance of $\mathrm{C}=\mathrm{C}$ (in $\mathrm{Y})$ and appearance of $\mathrm{C}=\mathrm{O}($ in $\mathrm{Y})$
OR
absence of peak at $1650\left(\mathrm{~cm}^{-1}\right)$ as no longer any $C=C$ present (in $Y$ )
AND
appearance of peak (in $Y$ ) at (any value / range of values) between $1670-1740\left(\mathrm{~cm}^{-1}\right)$ due to $\mathrm{C}=\mathrm{O}$
6(f)(i) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}+4[\mathrm{H}] \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}+\mathrm{H}_2 \mathrm{O}$
6(f)(ii) propan-1-ol
ALLOW propan-2-ol as error carried forward from 6f(i)
6(g)(i) Molecular formula of $\boldsymbol{W}$ $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}_2$
6(g)(ii) Possible structure of $\boldsymbol{W}$ $\mathrm{CH}_3 \mathrm{COOCH}_3$ OR $\mathrm{HCOOCH}{ }_2 \mathrm{CH}_3$