Question
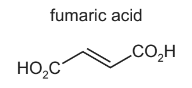
Fumaric acid is a naturally occurring dicarboxylic acid.
(a) Identify the products of the reaction between fumaric acid and an excess of hot, concentrated, acidified manganate(VII).
(b) Fumaric acid can form addition and condensation polymers.
(i) Draw the repeat unit of the addition polymer poly(fumaric acid).
(ii) Draw the repeat unit of the polyester formed when fumaric acid reacts with ethane-1,2-diol,
(CH2OH)2.
The ester bond should be shown fully displayed.
(iii) Explain why polyesters normally biodegrade more readily than polyalkenes.
(c) Fumaric acid reacts with cold, dilute, acidified manganate(VII) to form compound P.
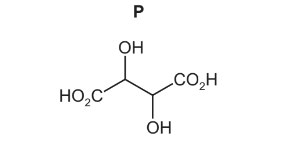
Only three stereoisomers of P exist. One of the stereoisomers is shown.
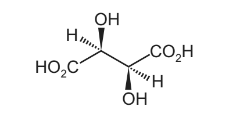
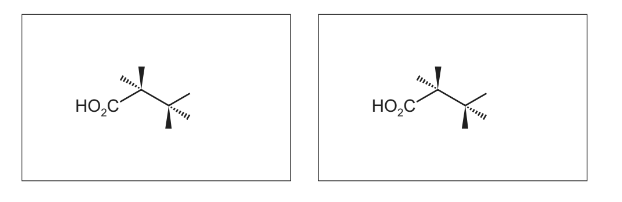
Complete the three-dimensional diagrams in the boxes to show the other two stereoisomers of P.
(d) The enzyme fumarase catalyses the reaction of fumarate ions, C4H2O42-, with water to form malate ions, C4H4O52-.
\(C_{4}H_{2}O_{4}^{2-} + H_{2}O \rightleftharpoons C_{4}H_{4}O_{5}^{2-}\)
Describe, with the aid of a suitably labelled diagram, how an enzyme such as fumarase can catalyse a reaction.
Answer/Explanation
Answer: (a) CO2 and H2O / in words
(b)(i) 
(b)(ii) 
(b)(iii) C—C bonds are non-polar / polyalkenes cannot be hydrolysed
OR polyesters / they can be broken down by hydrolysis
(c) 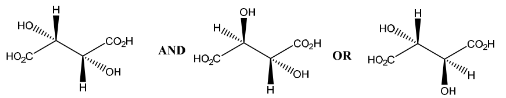
(d) 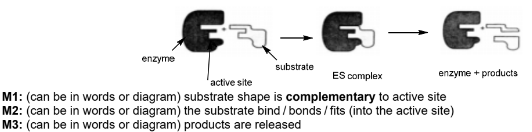
Question
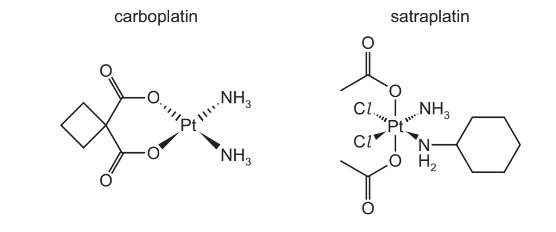
(a) Carboplatin and satraplatin are used as anticancer drugs instead of cisplatin.
(i) Describe the action of cisplatin as an anticancer drug.
(ii) Suggest the geometry of the platinum centre in the carboplatin complex.
(iii) Suggest why carboplatin does not show cis-trans isomerism.
(iv) Satraplatin is a neutral complex, containing the ligands CH 3CO2–, C6H11NH2, Cl– and NH3.
Deduce the oxidation state of platinum in satraplatin.
(b) Compound M is made from 1,3-dimethylbenzene in a two-step synthesis.
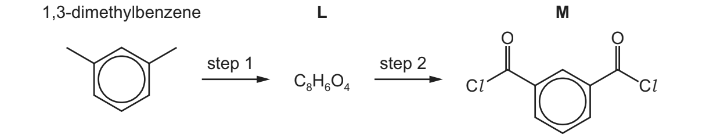
(i) Draw the structure of L.
(ii) Suggest reactants and conditions for each step of this synthesis.
(iii) Write an equation for step 2.
(iv) A student investigates a possible synthesis of M directly from benzene using COCl2 in the
presence of an AlCl3 catalyst.
Benzene initially reacts with COCl2 as shown.
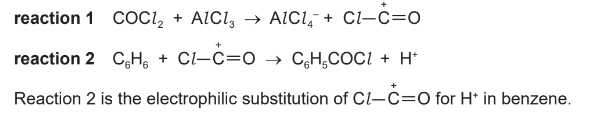
Suggest a mechanism for reaction 2.
Answer/Explanation
Answer: (a)(i) M1: (cisplatin) can bond / bind with DNA / (nitrogenous) base A, T, C, G etc.
M2: which prevents replication (of the DNA / strand)
OR prevents cell division / prevents mitosis
OR prevents transcription (and formation of mRNA)
(a)(ii) square planar
(a)(iii) the distance between two coordinating oxygens is too small to bond trans
OR atoms in a bidentate ligand can only bond 90° not 180°
(a)(iv) +4
(b)(i) 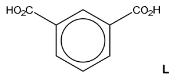
(b)(ii) M1: heat / reflux with acidified / alkaline KMnO4 (then acidify)
M2: PCl5OR SOCl2/ (heat with) PCl3
(b)(iii) ![]()
(b)(iv) 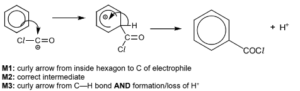 D
D