Question
During the bromination of methane, the free radical $\mathrm{CH}_3 \cdot$ is generated. A possible termination step of this reaction is the formation of $\mathrm{C}_2 \mathrm{H}_6$ by the combination of two free radicals.
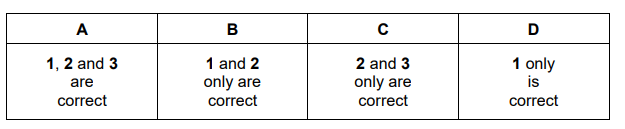
What could be produced in a termination step during the bromination of propane?
$1 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CH}_2 \mathrm{CH}_3$
$2 \mathrm{CH}_3 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CH}\left(\mathrm{CH}_3\right)_2$
$3 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}\left(\mathrm{CH}_3\right)_2$
▶️Answer/Explanation
Ans:C
Question:
Which statements comparing ethene and ethane are correct?
1 The bond angles in ethene are larger than the bond angles in ethane.
2 Ethene reacts much more quickly with bromine in the dark than ethane does.
3 Complete combustion of 0.01 mol of ethene or ethane produces the same volume of gas measured at room temperature and pressure

▶️Answer/Explanation
Ans:A
Question
What is the least number of carbon atoms in a non-cyclic alkane molecule that has a chiral centre?
A 7 B 8 C 9 D 10
Answer/Explanation
Ans: A
Question
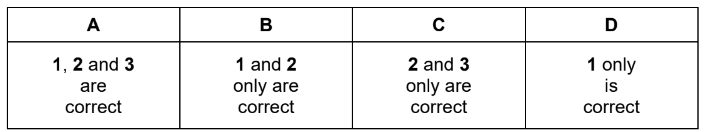
One molecule of dodecane, C12H26, is cracked, producing three product molecules, X, Y and Z.
X is a straight chain alkane. Y and Z are straight chain alkenes with different Mr values.
Which statements about X, Y and Z are correct?
1 If Y and Z are but-1-ene and ethene respectively, X will be hexane.
2 If X is butane, then Y and Z could both show cis-trans isomerism.
3 X could be octane.
▶️Answer/Explanation
Answer D
Question
Many reactions take place in the engine and catalytic converter of a car. Which pair of substances is produced both by the reactions in a car engine and in a catalytic converter?
A carbon dioxide and unburnt hydrocarbons
B carbon dioxide and water
C carbon monoxide and nitrogen
D carbon monoxide and unburnt hydrocarbons
Answer/Explanation
Answer:
B
Question
Alkanes are saturated hydrocarbons.
Which type of reaction are alkanes most likely to undergo?
A electrophilic addition
B electrophilic substitution
C free radical substitution
D nucleophilic addition
Answer/Explanation
Answer C
Question
Which reagent could detect the presence of alcohol in a mixture consisting mainly of alkanes and alkenes?
A Na
B Br2 (in CCl4)
C KMnO4(aq)
D 2,4-dinitrophenylhydrazine
Answer/Explanation
Answer A
Question
Which equation correctly represents the balanced equation for the complete combustion of a hydrocarbon with the formula\( C_{x}H_{y}\)?
A \(C_{X}H_{Y}+(X+\frac{y}{2})O_{2}\rightarrow XCO_{2}+\frac{y}{2}H_{2}O\)
B\(C_{X}H_{Y}+(X+\frac{y}{4})O_{2}\rightarrow XCO_{2}+yH_{2}O\)
C\( C_{X}H_{Y}+(X+\frac{y}{4})O_{2}\rightarrow XCO_{2}+\frac{y}{4}H_{2}O\)
D\( C_{X}H_{Y}+(X+\frac{y}{4})O_{2}\rightarrow XCO_{2}+\frac{y}{2}H_{2}O\)
Answer/Explanation
Ans:D
Question
Aluminium carbide, \(Al_{ 4}C_{3}\), reacts readily with aqueous sodium hydroxide. The two products of the reaction are \(NaAlO_{2}\) and a hydrocarbon. Water molecules are also involved as reactants. What is the formula of the hydrocarbon?
A CH_{4} B \(C_{2}H_{6} \) C \( C_{3}H_{8}\) D \(C_{6}H_{12}\)
Answer/Explanation
Ans:B
Question
Pentane, \(C_5H_{12}\), is reacted with chlorine in the presence of ultraviolet light. A compound R is
found in the products. R has molecular formula \(C_5H_{10}Cl_2\). Each molecule of R contains one chiral carbon atom.
Which two atoms of the pentane chain could be bonded to chlorine atoms in this isomer?
A 1 and 3 B 1 and 5 C 2 and 3 D 2 and 4
Answer/Explanation
Ans: A
Question
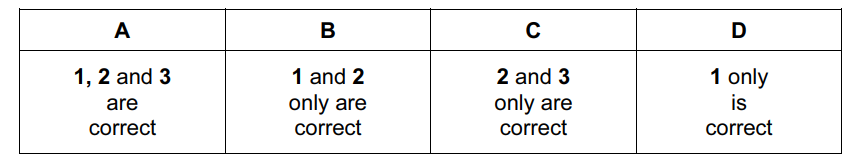
Chloroethane can be formed from bromoethane in two steps.
\(C_{2}H_{5}Br\overset{step X}{\rightarrow}C_{2}H_{5}OH\overset{step Y}{\rightarrow}C_{2}H_{5}Cl \)
Which statements about these steps are correct?
- Step X involves nucleophilic substitution.
- Hot aqueous sodium hydroxide is the reagent in step X.
- Hot aqueous sodium chloride is the reagent in step Y.
Answer/Explanation
Ans:
B
Question
The cracking of a single hydrocarbon molecule, CnH2n+2, produces two hydrocarbon molecules only. Each hydrocarbon product contains the same number of carbon atoms in one molecule. Each hydrocarbon product has non-cyclic structural isomers.
What is the value of n?
A 4 B 6 C 8 D 9
Answer/Explanation
Ans:
C