Question
(a) Long chain alkanes such as 4-methylheptane can be ‘cracked’ to produce shorter chain hydrocarbons.
(i) State the conditions necessary for this reaction to take place.
(ii) Suggest the structure of B.
(iii) Compounds C, D and E are isomers with the molecular formula \(C_{5}H_{10}\).
On heating with concentrated acidifi ed \(KMnO_{4}\),
● compound C gives \(CO_{2}\) and compound F \((C_{4}H_{8}O_{2})\),
● D and E each give a 1 : 1 mixture of compounds G \((C_{2}H_{4}O_{2})\) and \(H (C_{3}H_{6}O_{2})\). Suggest structures for compounds C – H.
(iv) Name the type of isomerism shown between D and E.
(b) Propene, \(CH_{3}CH=CH_{2}\), reacts with bromine to give 1,2-dibromopropane.
(i) How is this reaction usually carried out?
(ii) State the type of reaction that is occurring here.
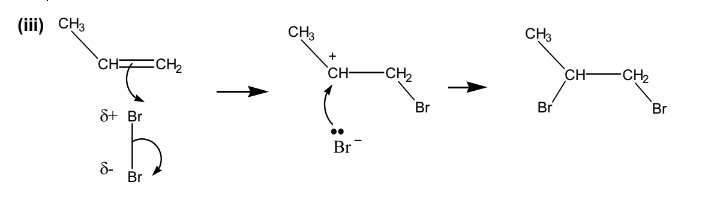
(iii) Draw the mechanism of this reaction, including the structures of any intermediates, and any dipoles, lone pairs and curly arrows to show the movements of electrons.
Answer/Explanation
(a) (i) heat with catalyst or heat with\( Al_2O_3\) /SiO_2 \)
(ii) B is\( CH_3CH_2CH_3 \)
(iii) C is \(CH_2\)=CHCH_2CH_2C_3\) D and E are \(CH_3CH\)= \(CHCH_2CH_3\) (one shown as cis, the other as trans) F is\ (CH_3CH_2CH_2CO_2\)H
G is\( CH_{3}CO_{2}\)H
H is\ (CH_3}CH_{2}CO_{2}H\)
(iv) geometrical or cis-trans or E–Z
(b) (i) No particular conditions or in the dark
(ii) electrophilic addition
Question
Alkanes and alkenes both react with bromine.
(a) Explain how and why bromine can be used to distinguish between an alkene and an alkane.
(b) The reaction of ethane with bromine forms a mixture of products.
(i) State the essential conditions for this reaction to occur.
(ii) Give the full name of the mechanism of this reaction.
(iii) Give the equation for a termination step that could occur, producing a hydrocarbon.
(iv) Give the equation for one propagation step involved in the formation of dibromoethane
from bromoethane during this reaction.
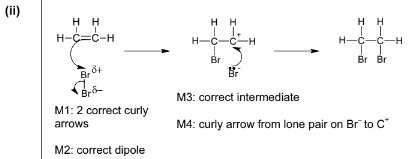
(c) The reaction of ethene with bromine forms a single product.
(i) Give the full name of the mechanism of this reaction.
(ii) Complete the diagram below to illustrate this mechanism. Include all relevant charges, partial charges, curly arrows and lone pairs.
![]()
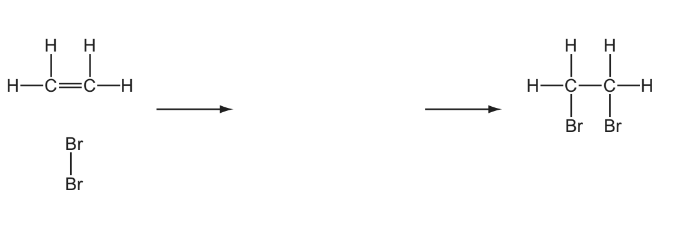
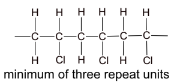
(d) Chloroethene can be polymerised to form a polymer commonly known as PVC. Draw a diagram of the structure of PVC including three repeat units.
(e) Chloroethane undergoes a series of reactions as shown in the diagram below.
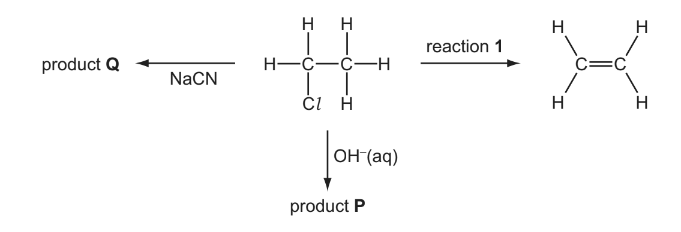
(i) Give the reagent and conditions necessary for reaction 1.
(ii) Give the skeletal formula of product P.
(iii) Give the displayed formula and the name of product Q.
Answer/Explanation
(a) decolourisation with an alkene at room conditions / quickly / easily /
OR alkane needs higher temp/UV/ is slow at room conditions double/ π /pi bond/C =C present in alkenes
(b) (i) UV light/ sunlight/ high temperature
(ii) (Free) radical
Substitution
(iii) \(•C_{2}H_{5} + •C_{2}H_{5} \rightarrow C_{4}H_{10}\)
(iv) \(C_{2}H_{5}Br + Br^{•}\rightarrow •C_{2}H_{4}Br + HBr\) OR
\(•C2H4Br + Br_{2} \rightarrow C_{2}H_{4}Br_{2} + Br^{•}\)
(c) (i) Electrophilic
Addition
(d)
(e) (i) NaOH/KOH
ethanolic / alcoholic AND heat/reflux
(ii)![]()
(iii) 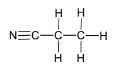
Propanenitrile/propanonitrile/ propionitrile/ ethyl cyanide/ cyanoethane