Question
In recent years there has been worldwide interest in the possible extraction of ‘shale gas’ (a form of natural gas) as an important energy source.
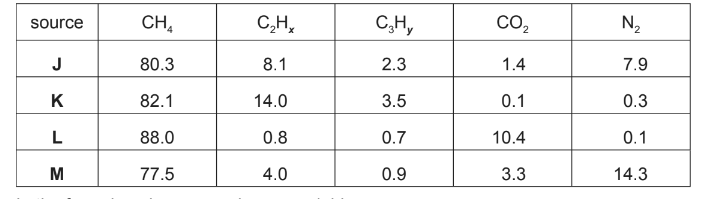
(a) One of the problems associated with using shale gas is its variable composition. Table 1 shows the percentage composition of shale gas from four different sources J, K, L and M.
In the formulae above, x and y are variables.
Table 1
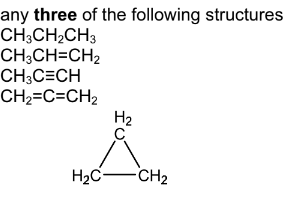
(i) Draw the structures of three possible compounds with the formula \(C_{3}H_{y}\).
(ii) Which source of shale gas, J, K, L or M, will provide the most energy when burned?
Explain your answer.
(iii) Suggest two methods by which carbon dioxide can be removed from shale gas.
1
2
(b) Table 2 shows a comparison of the relative amounts of pollutants produced when shale gas,
fuel oil and coal are burned to produce the same amount of energy.
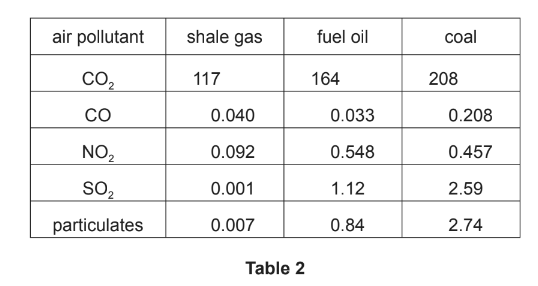
(i) Suggest why shale gas produces the smallest amount of \(CO_{2}\).
………………………………………..
(ii) Explain which of the three fuels, shale gas, fuel oil or coal, is the largest contributor to ‘acid rain’. fuel
(iii) Suggest a reason why fuel oil and coal produce more\( NO_{2}\) than shale gas.
(iv) State one environmental consequence of raised levels of
● CO,
● \(CO_{2}\).
Answer/Explanation
(a) (i)
(ii) K
since it has the greatest % of hydrocarbons / carbon-containing compounds
or 99.6 % of it is burnt for energy
(iii) any two from
• reacted with lime/CaO/ soda lime\(/Ca(OH)_{2}\) /KOH/NaOH/
• liquefied under pressure/ ≥5 atm
• dissolved in water under pressure/ ≥5 atm
(b) (i) have a shorter carbon/ hydrocarbon chain or shorter hydrocarbon or fewer carbon atoms in its chain or have high H/C ratio
(ii) Coal
produces the largest amount of \(SO_{2}\) or largest combined amount of \(SO_{2}\) and\( NO_{2}\)
(iii) they burn at higher temperatures
or release more heat on burning
(iv) CO – the gas is toxic/poisonous or references to Hb and ability to carry oxygen \(CO_{2}\) – the gas contributes to global warming
Question
Boron forms many useful compounds.
(a) The compound diborane, \(B_{2}H_{6}\), can be used as a rocket fuel.
It can be prepared by the reaction of boron trifluoride, \(BF_{3}\), with sodium borohydride, \(NaBH_{4}\).
Balance this equation.
…….\(BF_{3}\) + …….NaBH_{4} → …….B_{2}H6 + …….\(NaBF_{4}\)
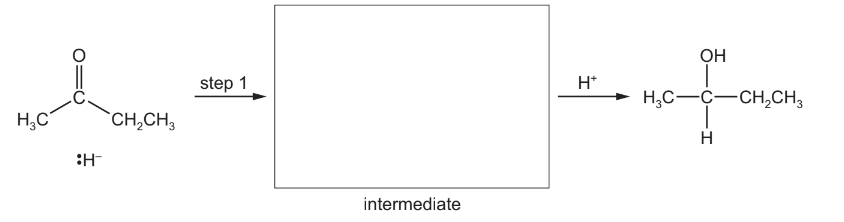
(b) Primary and secondary alcohols can be formed by the reaction of carbonyl compounds with
\(NaBH_{4}\), which is a source of hydride ions, \(H^{–}\)
. Complete the mechanism for the reaction of butanone with hydride ions, \(H^{–}\) , and draw the intermediate in the box. Include all necessary curly arrows and relevant dipoles.
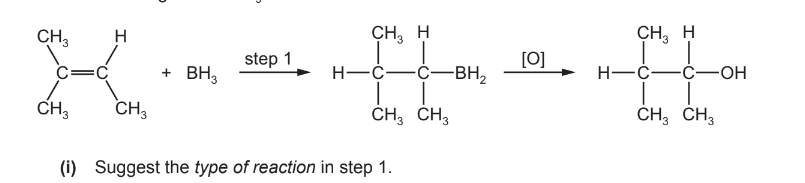
(c) Borane, \(BH_{3}\), is used to synthesise alcohols from alkenes. The reaction occurs in two steps. The \(BH_{2}\) group from \(BH_{3}\) bonds to the least substituted carbon atom of the double bond, and the remaining H from \(BH_{3}\) bonds to the other carbon.
(ii) The diol Y can be prepared by the same method.
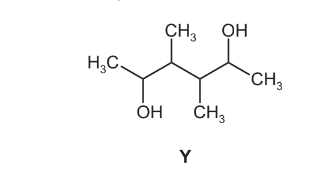
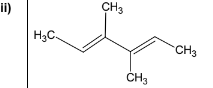
Draw the structure of the diene which could be used to prepare diol Y.
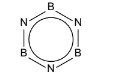
(d) Benzene,\( C_{6}H_{6}\), and borazine, \(B_{3}N_{3}H_{6}\), have planar, cyclic structures.
(i) Describe the structure of and bonding in benzene, \(C_{6}H_{6}\).
(ii) In borazine, \(B_{3}N_{3}H_{6}\), the boron and nitrogen atoms alternate around the ring. Each ring atom has a single hydrogen atom bonded to it.
All boron-nitrogen bonds in borazine are 0.144nm in length, whereas in simple compounds
B–N and B=N bond lengths are 0.154nm and 0.136nm respectively. Suggest and draw the structure of borazine.
Answer/Explanation
(a) \( 4BF_{3} + 3NaBH_{4}\) → \(2B_{2}H6 + 3NaBF_{4}\)
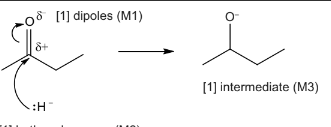
[1] both curly arrows (M2)
arrow must come from lone pair
(c) (i) (electrophilic) addition
(d) (i) any four of
M1:
σ-bonds between C–C or C–H
M2: π-bonds formed from overlap of p-orbitals
M3: (π-bonds/electrons) above and below the ring
M4:bonds/electrons are delocalised
M5: bond angle 120°
M6: intermediate C–C bond length/all C–C same length/ strength
M7: carbons are \(sp^{2}\) hybridised
(ii) correct delocalised structure of borazine