Question
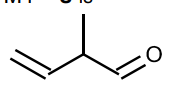
Some reactions of compound $\mathbf{P}, \mathrm{C}_5 \mathrm{H}_8 \mathrm{O}$, are shown.
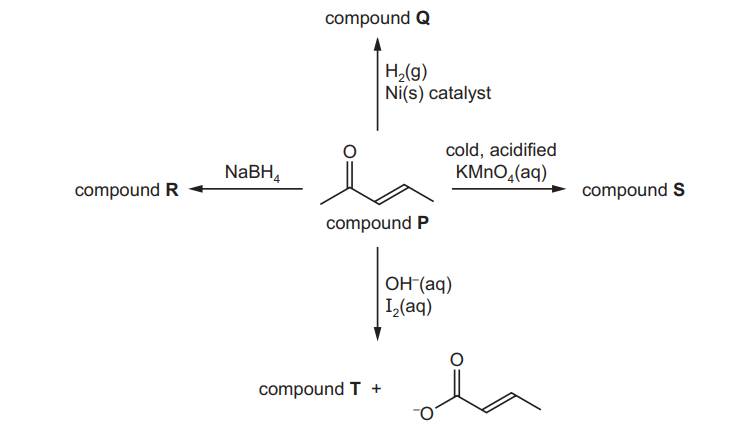
(a) (i) Give the structures for organic compounds Q, R, S and T.
(ii) Give the systematic name of compound $\mathbf{P}$.[1]
(iii) What would you observe when compound $\mathbf{P}$ is reacted with 2,4-dinitrophenylhydrazine $(2,4-\mathrm{DNPH})$ ?[1]
(b) Compound $\mathbf{U}$ contains a chiral centre and has the same molecular formula as compound $\mathbf{P}$, $\mathrm{C}_5 \mathrm{H}_8 \mathrm{O}$.
- Compound $\mathbf{U}$ readily decolourises a sample of bromine water.
- Compound $\mathbf{U}$ does not show cis-trans isomerism.
- When compound $\mathbf{U}$ is heated under reflux in the presence of excess acidified potassium dichromate(VI), the organic product gives the infra-red spectrum shown.
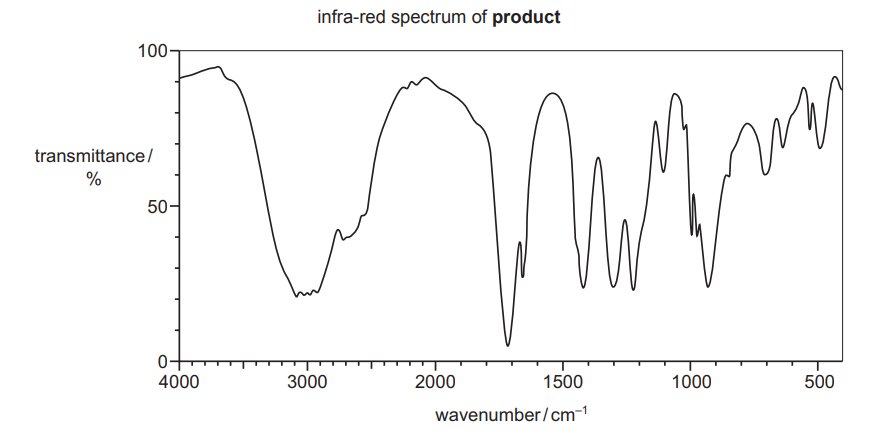
Use the information given to suggest a structure for compound U.
Explain your answer.
▶️Answer/Explanation
Ans:
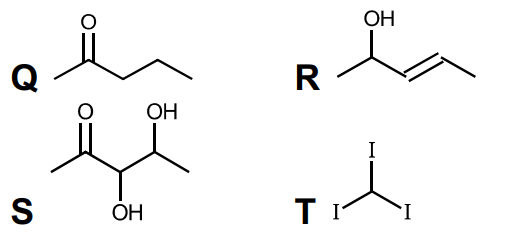
(ii) pent-3-en(e)-2-one
OR
3-penten-2-one
(iii) red/ orange/ yellow precipitate/ solid
(b) $\quad$ This question was discounted.
M1 = decolourises bromine $/ 1500-1600 \mathrm{~cm}^{-1}=$ alkene
M2 = absorption at $1700 \mathrm{~cm}^{-1}$ is $\mathrm{C}=\mathrm{O}$
AND
(very) broad absorption at $2500-3000 \mathrm{~cm}^{-1}$ is $\mathrm{O}-\mathrm{H}=$ carboxylic acid M3 = no cis-trans so terminal alkene OR chiral so contains a carbon atom with 4 different groups attached $\mathrm{M} 4=\mathbf{U}$ is
Question
(a) A series of reactions starting from 1-bromobutane is shown.
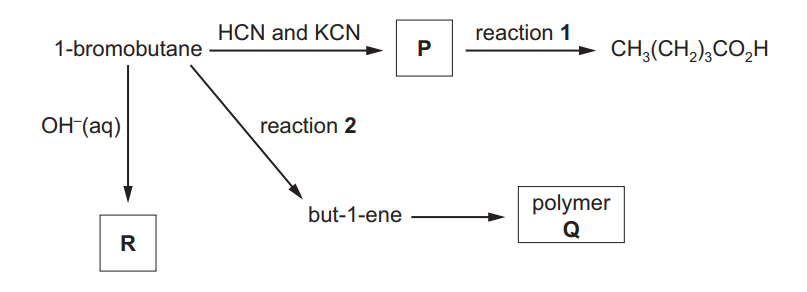
(i) Draw the displayed formula of compound P
(ii) Identify the reagent(s) and conditions for reactions 1 and 2.
reaction 1 …………………………………………………………………………………………………………….
reaction 2 ……………………………………………………………………………………………………………. [2]
(iii) Draw the structure of the repeat unit of polymer Q.
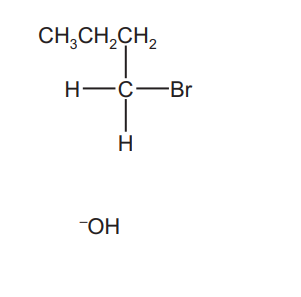
(b) Complete the reaction scheme to show the mechanism of the reaction of 1-bromobutane with $\mathrm{OH}^{-}(\mathrm{aq})$ to produce $\mathbf{R}$.
Include all necessary charges, dipoles, lone pairs and curly arrows and the structure of $\mathbf{R}$.
(c) But-1-ene reacts with steam as shown to form a mixture of two structural isomers, S and T.
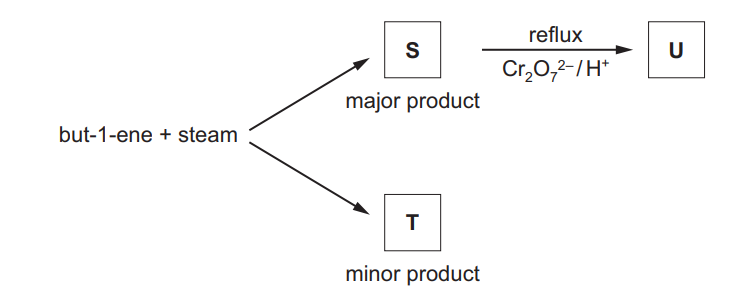
S can be oxidised with acidified potassium dichromate(VI) to form compound U.
S and U both react with alkaline aqueous iodine.
(i) Identify the type of reaction that occurs when but-1-ene reacts with steam.
……………………………………………………………………………………………………………………… [1]
(ii) State what can be deduced about the structure of S from its reaction with alkaline aqueous iodine.
……………………………………………………………………………………………………………………… [1]
(iii) Explain why S is the major product of the reaction of but-1-ene with steam
(iv) Draw the skeletal formulae of S, T and U
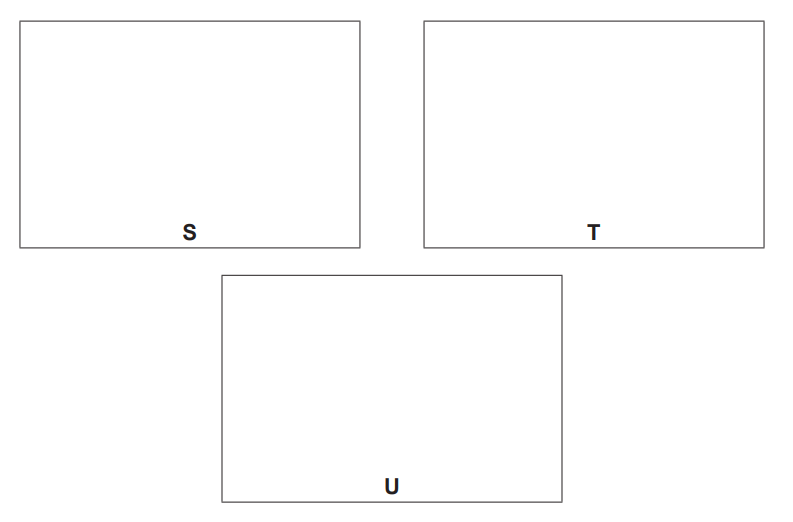 [3]
[3]
(v) Write an equation to represent the oxidation of S to U by acidified potassium dichromate(VI).
You should use [O] to represent the oxidising agent.[1]
(d) $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$ is a colourless liquid with an unpleasant odour.
It reacts with methanol in the presence of an acid catalyst to produce an organic product $\mathbf{V}$, which has a pleasant fruity smell.
(i) Name V. [1]
(ii) A student analysed $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$, methanol and $\mathbf{V}$ using infra-red spectroscopy. The spectra were returned to the student without labels.
Identify which of the infra-red spectra, $\mathrm{X}, \mathrm{Y}$ or $\mathrm{Z}$, corresponds to $\mathbf{V}$.
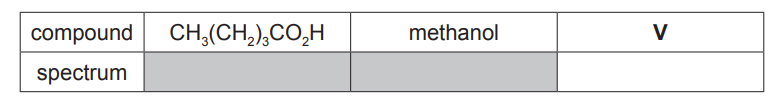
Explain your answer with reference to relevant features of the three spectra in the region above $1500 \mathrm{~cm}^{-1}$. [4]
▶️Answer/Explanation
Ans:
(a)(i)
(a)(ii) reaction 1 = HCl(aq)
reaction 2 = (conc.) NaOH/KOH AND ethanol
(a)(iii)
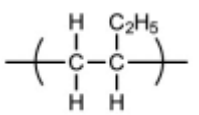
C–C backbone with dangling bonds
rest of structure
(b)
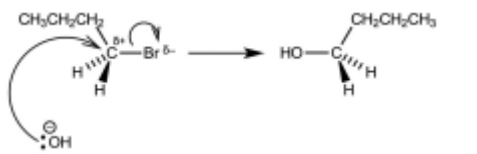
lone pair on O AND curly arrow from O to C of C–Br
dipole on C–Br AND curly arrow from C–Br to Br
product (butan-1-ol)
(c)(i) (electrophilic) addition
(c)(ii) $\quad \mathbf{S}$ has $\mathrm{CH}_3 \mathrm{CHOH}$ OR methyl/ $\mathrm{CH}_3$ group next to $\mathrm{CHOH}$
(c)(iii) positive inductive effect of more alkyl groups /more alkyl groups donate electron density
secondary carbocation / secondary intermediate is more stable (than primary)
(c)(iv)
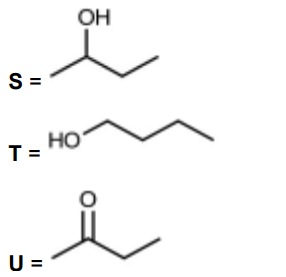
$(c)(v) \quad \mathrm{CH}_3 \mathrm{CHOHCH}_2 \mathrm{CH}_3+[\mathrm{O}] \rightarrow \mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{CH}_3+\mathrm{H}_2 \mathrm{O}$
(d)(i) methyl pentanoate
(d)(ii) (compound V is) spectrum X
spectra $X$ and $Z$ show a $C=O$ (stretch) at $1730\left(\mathrm{~cm}^{-1}\right)$
spectra $Y$ and $Z$ show O-H (stretches) above $2500\left(\mathrm{~cm}^{-1}\right)$
$\mathbf{V}$ has a $\mathrm{C}=\mathrm{O}$ (bond) and no $\mathrm{O}-\mathrm{H}$ (bond)