Question
Over one million tonnes of hydrogen cyanide, $\mathrm{HCN}$, are produced each year using the Andrussow process. The overall equation for the reaction is shown.
$
\mathrm{CH}_4(\mathrm{~g})+\mathrm{NH}_3(\mathrm{~g})+1 \frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightleftharpoons \mathrm{HCN}(\mathrm{g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
$
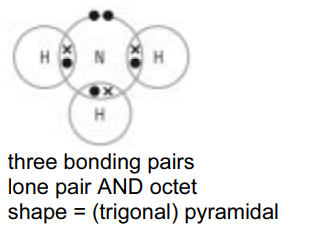
(a) (i) Draw a dot-and-cross diagram to represent the bonding in a molecule of ammonia, $\mathrm{NH}_3$, and state the shape of the molecule.
shape of molecule[3]
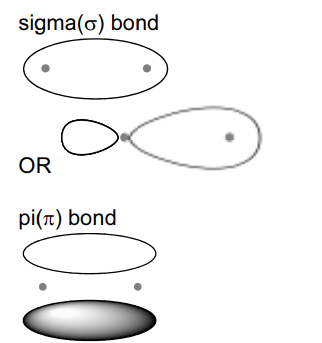
(ii) A molecule of hydrogen cyanide, $\mathrm{HCN}$, is shown.
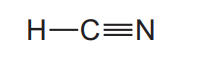
The bonding between the carbon and nitrogen atoms consists of one sigma (σ) bond andtwo pi (π) bonds.
Sketch the shape of the sigma bond and one of the pi bonds in the space below.
Show clearly the position of the atomic nuclei in each diagram.
(b) The reaction exists as a dynamic equilibrium.
(i) Explain what is meant by the term dynamic equilibrium……………………………………………………………………………………………………………………………. [1]
(ii) State and explain how the amounts of the chemicals present in the equilibrium mixture will
change when the pressure is increased………………………………………………………………………………………………………………………. [2]
(c) The process uses a platinum catalyst, which increases the rate of reaction.
Sketch a Boltzmann distribution on the axes given below and use your diagram to explain how the platinum catalyst increases the rate of the reaction.
 [3]
[3]
(d) The reaction of hydrogen cyanide with propanone is an important first step in many organic syntheses.
(i) Give the full name of the mechanism of this reaction.
……………………………………………………………………………………………………………………… [1]
(ii) Complete the diagram to show the mechanism of the reaction of hydrogen cyanide with propanone.
Draw the structure of the intermediate and the product of the reaction. Include all relevant charges, partial charges, curly arrows and lone pairs.
 [5][Total: 17]
[5][Total: 17]
▶️Answer/Explanation
Ans:
(a) (i)
(ii)
(b) (i) forward and backward reactions occurring at same rate OR the rate of forward and backward reactions are equal
(ii) $\mathrm{M} 1=$ decreased yield of products $/$ less products formed $/$ ora M2 = left-hand side has fewer moles of gas OR equilibrium shifts to the left
(c)
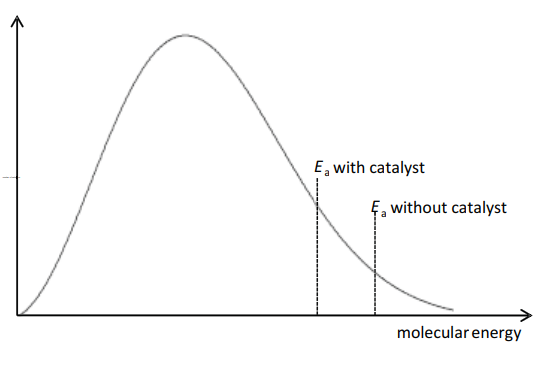
M1 = correct Boltzmann curve
M2, M3 any 2 from:
- line for both $E_a$ values or statement in text that catalyst lowers $E_a$
- (catalyst) increases proportion/number of molecules/particles with energy $\geqslant$ activation energy
- so more frequent successful collisions
(d) (i) nucleophilic addition
(ii)
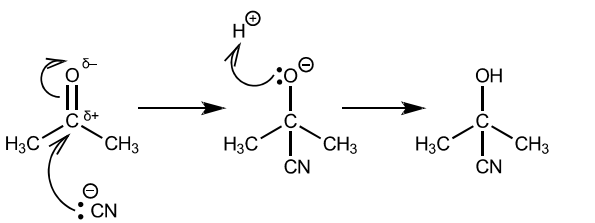
correct dipole on carbonyl
curly arrow from lone pair on $\mathrm{CN}^{-} \mathrm{AND}$ from $\mathrm{C}=\mathrm{O}$ to $\mathrm{O}$ correct intermediate curly arrow from lone pair on $\mathrm{O}^{-}$to $\mathrm{H}^{+}$ correct product
Question
(a) A series of reactions starting from 1-bromobutane is shown.
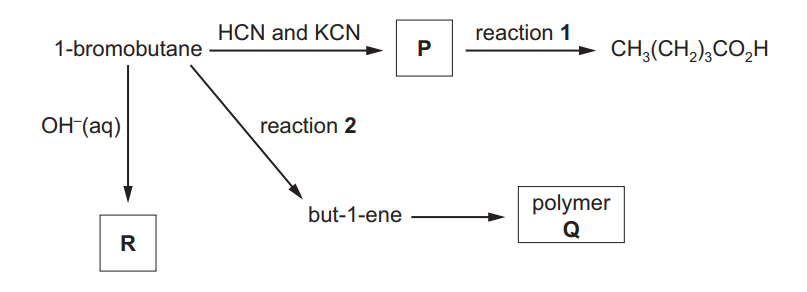
(i) Draw the displayed formula of compound P
(ii) Identify the reagent(s) and conditions for reactions 1 and 2.
reaction 1 …………………………………………………………………………………………………………….
reaction 2 ……………………………………………………………………………………………………………. [2]
(iii) Draw the structure of the repeat unit of polymer Q.
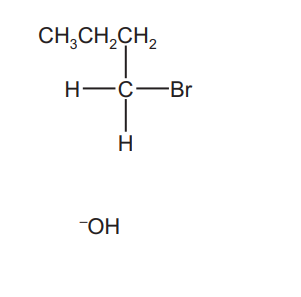
(b) Complete the reaction scheme to show the mechanism of the reaction of 1-bromobutane with $\mathrm{OH}^{-}(\mathrm{aq})$ to produce $\mathbf{R}$.
Include all necessary charges, dipoles, lone pairs and curly arrows and the structure of $\mathbf{R}$.
(c) But-1-ene reacts with steam as shown to form a mixture of two structural isomers, S and T.
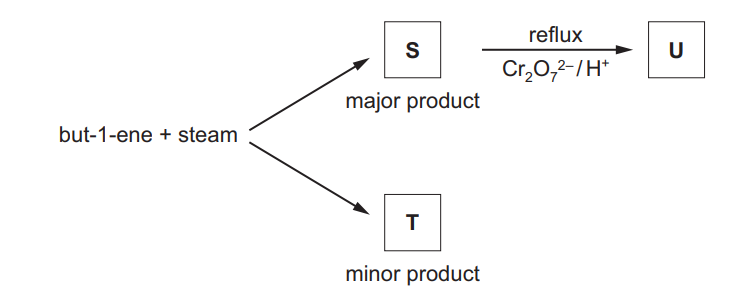
S can be oxidised with acidified potassium dichromate(VI) to form compound U.
S and U both react with alkaline aqueous iodine.
(i) Identify the type of reaction that occurs when but-1-ene reacts with steam.
……………………………………………………………………………………………………………………… [1]
(ii) State what can be deduced about the structure of S from its reaction with alkaline aqueous iodine.
……………………………………………………………………………………………………………………… [1]
(iii) Explain why S is the major product of the reaction of but-1-ene with steam
(iv) Draw the skeletal formulae of S, T and U
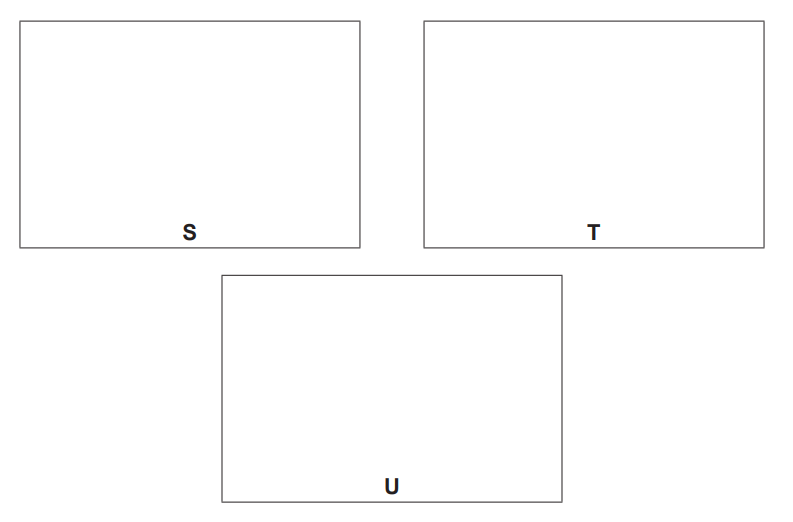 [3]
[3]
(v) Write an equation to represent the oxidation of S to U by acidified potassium dichromate(VI).
You should use [O] to represent the oxidising agent.[1]
(d) $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$ is a colourless liquid with an unpleasant odour.
It reacts with methanol in the presence of an acid catalyst to produce an organic product $\mathbf{V}$, which has a pleasant fruity smell.
(i) Name V. [1]
(ii) A student analysed $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$, methanol and $\mathbf{V}$ using infra-red spectroscopy. The spectra were returned to the student without labels.
Identify which of the infra-red spectra, $\mathrm{X}, \mathrm{Y}$ or $\mathrm{Z}$, corresponds to $\mathbf{V}$.
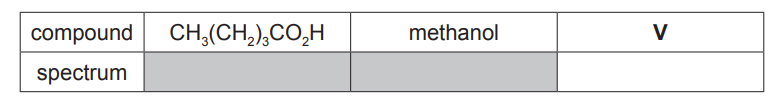
Explain your answer with reference to relevant features of the three spectra in the region above $1500 \mathrm{~cm}^{-1}$. [4]
▶️Answer/Explanation
Ans:
(a)(i)
(a)(ii) reaction 1 = HCl(aq)
reaction 2 = (conc.) NaOH/KOH AND ethanol
(a)(iii)
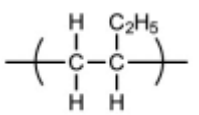
C–C backbone with dangling bonds
rest of structure
(b)
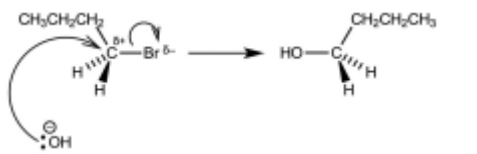
lone pair on O AND curly arrow from O to C of C–Br
dipole on C–Br AND curly arrow from C–Br to Br
product (butan-1-ol)
(c)(i) (electrophilic) addition
(c)(ii) $\quad \mathbf{S}$ has $\mathrm{CH}_3 \mathrm{CHOH}$ OR methyl/ $\mathrm{CH}_3$ group next to $\mathrm{CHOH}$
(c)(iii) positive inductive effect of more alkyl groups /more alkyl groups donate electron density
secondary carbocation / secondary intermediate is more stable (than primary)
(c)(iv)
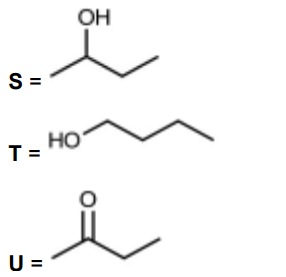
$(c)(v) \quad \mathrm{CH}_3 \mathrm{CHOHCH}_2 \mathrm{CH}_3+[\mathrm{O}] \rightarrow \mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{CH}_3+\mathrm{H}_2 \mathrm{O}$
(d)(i) methyl pentanoate
(d)(ii) (compound V is) spectrum X
spectra $X$ and $Z$ show a $C=O$ (stretch) at $1730\left(\mathrm{~cm}^{-1}\right)$
spectra $Y$ and $Z$ show O-H (stretches) above $2500\left(\mathrm{~cm}^{-1}\right)$
$\mathbf{V}$ has a $\mathrm{C}=\mathrm{O}$ (bond) and no $\mathrm{O}-\mathrm{H}$ (bond)