Question:
An excess of P reacts with Q, in the presence of concentrated sulfuric acid, to form R. Effervescence is seen when a piece of sodium is added to pure R. The structure of P is shown
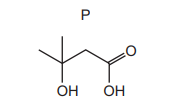
Which organic compounds could be compound Q?
▶️Answer/Explanation
Ans:B
Question:
Ethanedioic acid has the formula \(HO_{2}CCO_{2}H\). What is the formula of aluminium ethanedioate?
A \(A \imath C_{2}O_{4}\)
B \(A \imath (C_{2}O_{4})_{3}\)
C \(A \imath _{2}C_{2}O_{4}\)
D \(A\imath_{2}(C_{2}O_{4})_{3}\)
▶️Answer/Explanation
Ans:D
Question:
Which reaction gives butanoic acid as one of its products?
A acid hydrolysis of butyl ethanoate
B alkaline hydrolysis of butyl ethanoate
C acid hydrolysis of ethyl butanoate
D alkaline hydrolysis of ethyl butanoate
▶️Answer/Explanation
Ans:C
Question:
The structures of citric acid and isocitric acid are shown
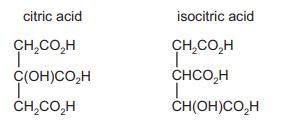
How many chiral centres does each acid possess?
▶️Answer/Explanation
Ans:D
Question
Which compound is chiral and reacts with $\mathrm{Na}_2 \mathrm{CO}_3$ to give $\mathrm{CO}_2$ ?
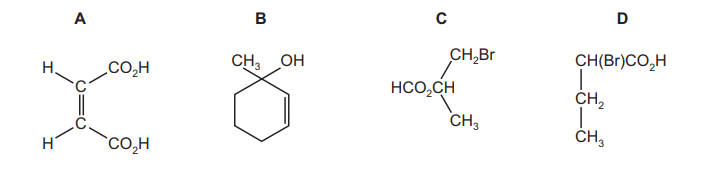
▶️Answer/Explanation
Ans:D
Question:
What is the skeletal formula of the compound formed when \(CH_{3}CH=CHCH_{2}OH\) is heated, under
reflux, with \(K_{2}Cr_{2}O_{7}/H^{+}\)?
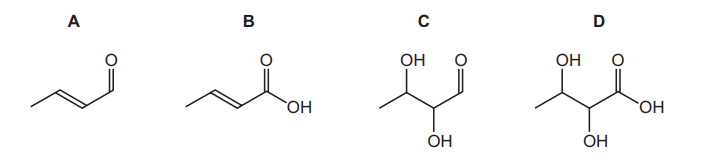
▶️Answer/Explanation
Ans:B
Question:
Compound X is treated with an excess of lithium aluminium hydride. The reaction is allowed to go to completion.
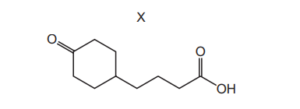
What is the structure of the organic product?
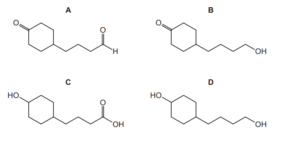
▶️Answer/Explanation
Ans:D
Question:
Which reaction would produce propanoic acid as one of its products?
A heating \((CH_{3})_{2}C=CHCH_{2}CH_{3}\) with concentrated, acidified \(kMnO_{4}\)
B heating \(CH_{3}CH_{2}CO_{2}CH_{2}CH_{2}CH_{3}\) with NaOH(aq)
C heating \(CH_{3}CH_{2}OH\) with acidified \(K_{2}Cr_{2}O_{7}\) under reflux
D reacting \(CH_{3}CHO\) with HCN then heating the organic product with \(H_{2}SO_{4}(aq)\)
▶️Answer/Explanation
Ans:A
Question:
The diagram shows the structure of ethanedioic acid.
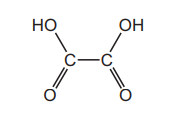
Ethanedioic acid reacts with ethanol in the presence of a few drops of concentrated sulfuric acid
to form a diester. The molecular formula of the diester is \(C_{6}H_{10}O_{4}\)
What is the structural formula of the diester?
A \(CH^{3}CH_{2}CO_{2}CO_{2}CH_{2}CH^{3}\)
B \(CH^{3}CH_{2}OCOCO_{2}CH_{2}CH_{3}\)
C \(CH_{3}CH_{2}O_{2}CO_{2}CCH_{2}CH_{3}\)
D \(CH_{3}CO_{2}CH_{2}CH_{2}OCOCH_{3}\)
▶️Answer/Explanation
Ans:B
Question
Propanoic acid occurs naturally as a result of the bacterial fermentation of milk and is partly responsible for the flavour of Swiss cheese.
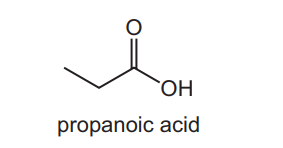
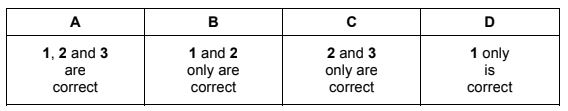
Which starting materials can be used to produce propanoic acid?
$1 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}$
$2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}$
$3 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CN}$
▶️Answer/Explanation
Ans:A
Question
An unknown organic compound $Z$ reacts with sodium to give a combustible gas as one product. $\mathbf{Z}$ does not give a yellow precipitate with alkaline aqueous iodine.
What is a possible identity of $\mathbf{Z}$ ?
1 ethanoic acid
2 pentan-3-ol
3 propan-1-ol
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
▶️Answer/Explanation
Ans:A
Question
The structure of tartaric acid is shown.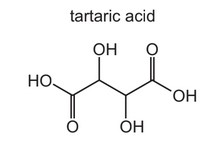
Four moles of substance X react with one mole of tartaric acid.
What could be substance X?
A sodium
B sodium carbonate
C sodium hydrogencarbonate
D sodium hydroxide
Answer/Explanation
Ans: A
Question
A student suggests two uses of \(LiAlH_4\).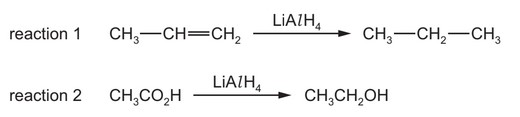
Which reactions would give the product shown?
A both reactions
B reaction 1 only
C reaction 2 only
D neither reaction
Answer/Explanation
Ans: C
Question
Which compound produces butan-2-ol and ethanoic acid on hydrolysis?
A \(CH_3CO_2CH(CH_3)_2\)
B \(CH_3CO_2CH(CH_3)CH_2CH_3\)
C \(CH_3CH(CH_3)CO_2CH_2CH_3\)
D \(CH_3CH_2CO_2CH(CH_3)CH_2CH_3\)
Answer/Explanation
Ans: B
Question
Which compound would produce two different carboxylic acids when treated with hot, concentrated, acidified manganate(VII) ions?
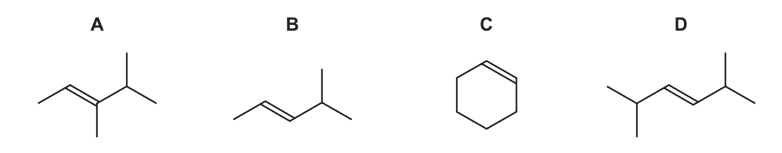
Answer/Explanation
Answer: B
Question
Butanoic acid is prepared from 1-bromopropane.
This synthesis requires a sequence of two reactions.
Which compound is prepared in the first stage of the synthesis?
A 1-aminopropane
B propan-1-ol
C butanal
D butanenitrile
Answer/Explanation
Answer: D
Question
How many moles of hydrogen, H2, are evolved when an excess of sodium metal is added to one mole of citric acid?
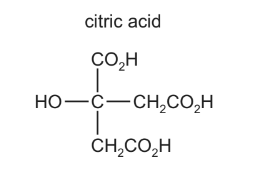
A 0.5 B 1.5 C 2 D 4
Answer/Explanation
Answer: C
Question
Propanoic acid is reacted with an excess of lithium aluminium hydride. The organic product of this reaction is reacted with ethanoic acid in the presence of concentrated sulfuric acid, forming product X.
What are major commercial uses of X?
1 fuel
2 solvent
3 flavouring
Answer/Explanation
Answer: C
Question
Citric acid is found in lemon juice.
citric acid
\(HO_{2}CCH_{2}C(OH)(CO_{2}H)CH_{2}CO_{2}H\)
Which volume of 0.40 mol dm–3 sodium hydroxide solution is required to neutralise a solution containing 0.0050 mol of citric acid?
A 12.5 cm3 B 25.0 cm3 C 37.5 cm3 D 50.0 cm3
Answer/Explanation
Answer C
Question
Compound L has the molecular formula C10H16.
A sample of L reacted with an excess of hot, concentrated, acidified potassium manganate(VII). Compound M is produced.
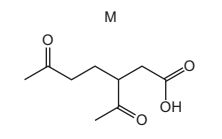
What could be the structure of compound L?
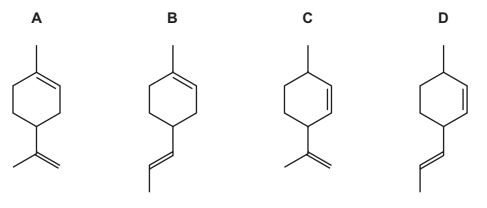
Answer/Explanation
Answer:
A
Question
When compound X is heated with Cr2O72– /H+ , a colour change from orange to green is observed.
Two tests are carried out on the organic product of this reaction.
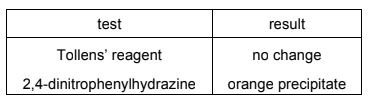
What could be compound X?
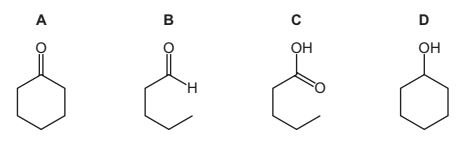
Answer/Explanation
Answer:
D
Question
Carboxylic acids can be prepared from alcohols, nitriles or esters.
Which statements are correct?
1 Both primary and secondary alcohols can be oxidised to carboxylic acids.
2 Carboxylic acids can be made from nitriles by hydrolysis.
3 Ethyl propanoate gives propanoic acid when reacted with hydrochloric acid.
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
C
Question
Tartaric acid, HO2CCH(OH)CH(OH)CO2H, is found in many plants.
A sample of tartaric acid reacts with an excess of LiAlH4 to form the organic product J.
What happens when NaOH(aq) is added to separate samples of tartaric acid and J?
A Both tartaric acid and J react.
B Only tartaric acid reacts.
C Only J reacts.
D Neither tartaric acid nor J react.
Answer/Explanation
Answer B
Question
Carboxylic acids can be made by several different reactions.
Which statements are correct?
1 The acid hydrolysis of CH3CH2CN will make ethanoic acid.
2 The oxidation of CH3CH2CH2CH2OH will make butanoic acid.
3 The oxidation of CH3CH2CHO will make propanoic acid.

Answer/Explanation
Answer C
Question
Carboxylic acids react with alcohols to produce esters.
Carboxylic acid X forms one ester only with molecular formula C5H10O2.
What could X be?
1 ethanoic acid
2 propanoic acid
3 butanoic acid

▶️Answer/Explanation
Answer C
Question
3-oxobutanoic acid can be synthesised in a two-step process.
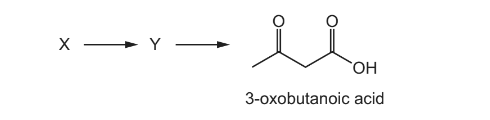
What could be the structure of X?
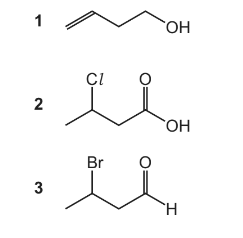
Answer/Explanation
Answer A
Question
Ethanedioic acid, HO2CCO2H, is reduced using an excess of lithium aluminium hydride, LiAlH4.
What is the organic product of the reaction?
A ethanol
B ethane-1,2-diol
C ethanedial, OHCCHO
D methane
Answer/Explanation
Answer B
Question
Which reagent could be used to carry out the following reaction?
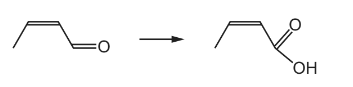
A a solution containing acidified dichromate(VI) ions
B a solution containing dilute, acidified manganate(VII) ions
C a solution containing hot, concentrated, acidified manganate(VII) ions
D concentrated sulfuric acid
Answer/Explanation
Answer A
Question
Four reactions of propanoic acid to form salts and other products are shown.
Which reaction does not show the formulae of all the correct products?
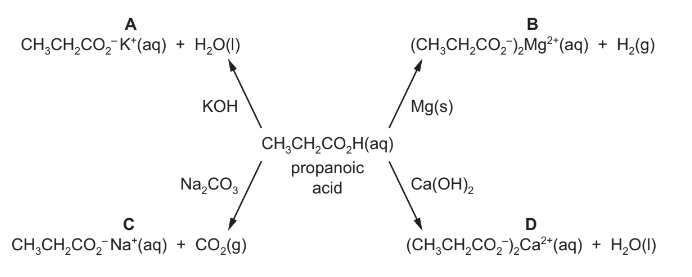
Answer/Explanation
Answer C
Question
The structure of lactic acid is shown.
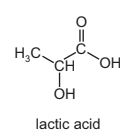
Which esters might form when lactic acid is heated?
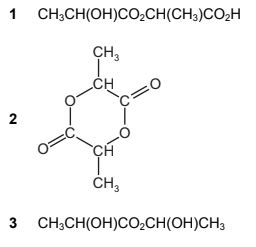
The responses A to D should be selected on the basis of
Answer/Explanation
Answer:
B
Question
Alcohols, aldehydes and nitriles can each be converted into carboxylic acids.
Which descriptions of their conversions into carboxylic acids are correct?
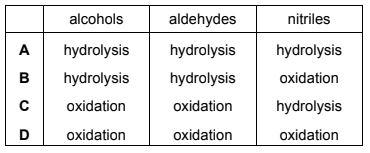
Answer/Explanation
Answer:
C
Question
Which compound reacts with 2,4-dinitrophenylhydrazine reagent but does not react with Tollens’ reagent?
A CH3COCO2H
B CH3CH(OH)CHO
C CH3COCHO
D CH3CH(OH)CH3
Answer/Explanation
Answer:
A
Question
Compound X can be converted into compound Y in a single step.
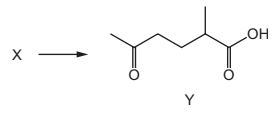
What could be the identity of X?
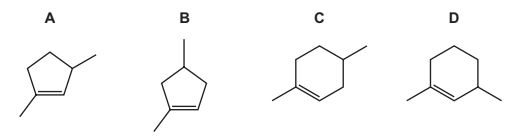
Answer/Explanation
Answer:
A
Question
At room temperature, propanoic acid was reacted to produce sodium propanoate. No gas was produced during the reaction.
What could the propanoic acid have reacted with?
A \(NaHCOP_3(aq)\) B \(NaOH(aq)\) C \(Na_2CO_3(aq)\) D \(Na_2SO_4(aq)\)
Answer/Explanation
Ans: B
Question
Citric acid can be converted into tricarballylic acid in two stages. An intermediate, Q, is formed.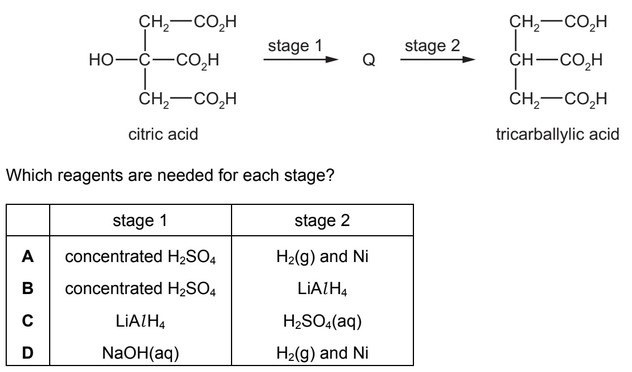
Answer/Explanation
Ans: A
Question
Which statements about ethanol and ethanoic acid are correct?
1 Both react with a suitable reagent to form an ester.
2 Both react with sodium.
3 Both are soluble in water.
The responses A to D should be selected on the basis of
Answer/Explanation
Ans: A
Question
Citral is found in lemongrass oil. It can react to give compound W.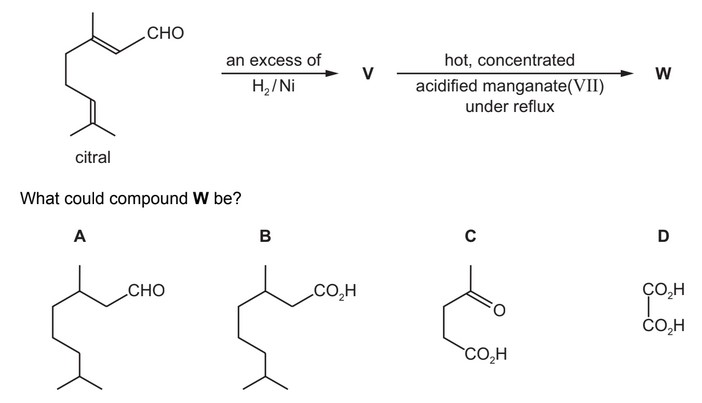
Answer/Explanation
Ans: B
Question
X and Y are the reagents required to convert 1-bromopropane into butanoic acid.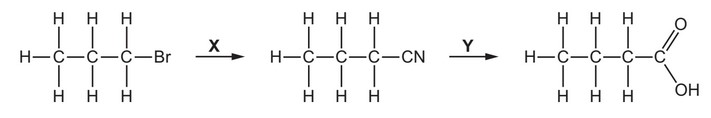
What are the correct identities of X and Y?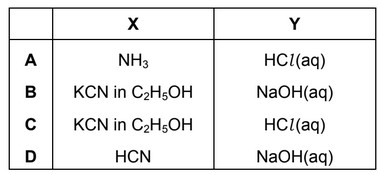
Answer/Explanation
Ans: C
Question
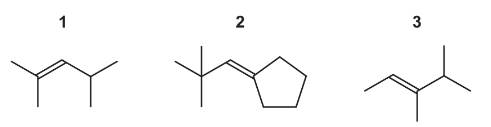
Answer/Explanation
Answer: A
Question
Which reaction would not give ethanoic acid?
A heating ethanenitrile under reflux with dilute sodium hydroxide
B heating ethanenitrile under reflux with dilute sulfuric acid
C heating ethanal under reflux with acidified sodium dichromate(VI)
D heating ethanol under reflux with acidified sodium dichromate(VI)
Answer/Explanation
Answer: A
Question
Several steps are involved in the synthesis of 2-hydroxypropanoic acid from ethanol.
C2H5OH → → → CH3CH(OH)CO2H
Which statements concerning this synthesis are correct?
1 The chain length can be increased during a step involving reaction between HCN and an
aldehyde.
2 The carboxyl group can be made by hydrolysis of a nitrile by boiling with NaOH(aq) and then
acidifying.
3 The ethanol should be first oxidised by heating it under reflux with an excess of acidified
potassium dichromate(VI).
Answer/Explanation
Answer: B
Question
Malic acid is found in apples.
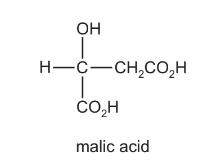
Which reagent will react with all three –OH groups present in the malic acid molecule?
A ethanol in the presence of concentrated sulfuric acid
B potassium hydroxide
C sodium
D sodium carbonate
Answer/Explanation
Answer: C
Question
The structural formula of compound X is shown below.
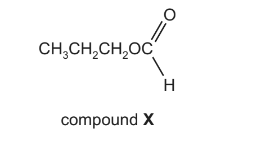
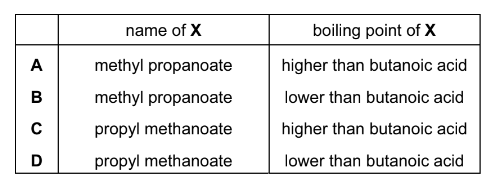
What is the name of compound X and how does its boiling point compare with that of butanoic
acid?
Answer/Explanation
Answer: D
Question
An organic compound, X, will react with calcium metal to produce a salt with the empirical formula \(CaC_{4}H_{4}O_{4}\).
What could be the identity X?
1 ethanoic acid
2 butanedioic acid
3 2-methylpropanedioic acid
The responses A to D should be selected on the basis of
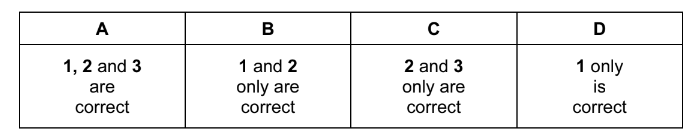
Answer/Explanation
Ans:C
Question
5-hydroxypentanoic acid is readily converted into the cyclic compound L.
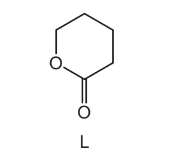
Which statements about this reaction are correct?
1 Acidified sodium dichromate(VI) is used as a reagent.
2 A water molecule is produced in the reaction.
3 The reaction is catalysed by concentrated \(H_{2}SO_{4}\).
The responses A to D should be selected on the basis of

Answer/Explanation
Ans:C
Question
Cottonseed oil contains large amounts of polyunsaturated carboxylic acids. When this oil is used M to make margarine, the C=C double bonds in the unsaturated carboxylic acids are hydrogenated. What reagents and conditions would be suitable to bring about this hydrogenation reaction?
A \(H_{2}\) gas, nickel catalyst, 400°C
B \( LiAlH_{4}\) in dry ether
C \( NaBH_{4}\), aqueous solution
D steam, concentrated \(H_{2}SO_{4}\), 300°C and 60atm pressure
Answer/Explanation
Ans:B
Quesrion
Which reactions result in the formation of propanoic acid?
1 \(CH_{3}CH_{2}CO_{2}\)Na with dilute H_{2}SO_{4}\)(aq)
2 \(CH_{3}CH=CHCH_{3}\) with hot, concentrated H+ / MnO4^{–}\)(aq)
3 \( CH_{3}CH_{2}OH with H^{+} /Cr_{2}O_{7}^{2–}(aq)\)
The responses A to D should be selected on the basis of
Answer/Explanation
Ans:D
Question
Which compound produces butan-2-ol and ethanoic acid on hydrolysis?
A \(CH_{3}CO_{2}CH(CH_{3})_{2}\)
B \( CH_{3}CO_{2}CH(CH_{3})CH_{2}CH_{3}\)
C\( CH_{3}CH(CH_{3})CO_{2}CH_{2}CH_{3}\)
D\( CH_{3}CH_{2}CO_{2}CH(CH_{3})CH_{2}CH_{3}\)
Answer/Explanation
Ans:B
Question.24
Lactic acid, \(CH_{3}CH(OH)CO_{2}H\ , causes pain when it builds up in muscles. Which reagent reacts with both of the –OH groups in lactic acid?
A acidified potassium dichromate(VI)
B ethanol
C sodium
D sodium hydroxide
Answer/Explanation
Ans:C
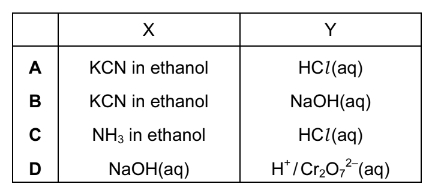
Question
Butanoic acid can be produced from 1-bromopropane using reagents X and Y as shown below.

What could be reagents X and Y?
Answer/Explanation
Ans:A
Question
Lactic acid (2-hydroxypropanoic acid), \(CH_3CH(OH)CO_2H\), is found in sour milk.
Which reaction could occur with lactic acid?
A \(CH_3CH(OH)CO_2H + CH_3OH → CH_3CH(OCH_3)CO_2H + H_2O\)
B \(CH_3CH(OH)CO_2H + HCO_2H → CH_3CH(O_2CH)CO_2H + H_2O\)
C \(CH_3CH(OH)CO_2H + NaHCO_3 → CH_3CH(ONa)CO_2H + H_2O + CO_2\)
D \(CH_3CH(OH)CO_2H + Cl_2 → CH_3CH(Cl)CO_2H + HOCl\)
Answer/Explanation
Ans: B
Question
How many moles of hydrogen, , are evolved when an excess of sodium metal is added to one
mole of citric acid?
A 1 B 2 C 3 D 4
Answer/Explanation
Ans: B
Question
Which reagent reacts with ethanol and also reacts with ethanoic acid?
- acidified potassium dichromate(VI)
- sodium
- sodium carbonate
- sodium hydroxide
Answer/Explanation
Ans:
B
Question
Which equations represent stages in the Contact process for manufacturing sulfuric acid?
- \(SO_{2}+\frac{1}{2}O_{2}\rightarrow SO_{3}\)
- \(SO_{2}+H_{2}O\rightarrow H_{2}SO_{3}\)
- \(H_{2}SO_{3}+\frac{1}{2}O_{2}\rightarrow H_{2}SO_{4}\)
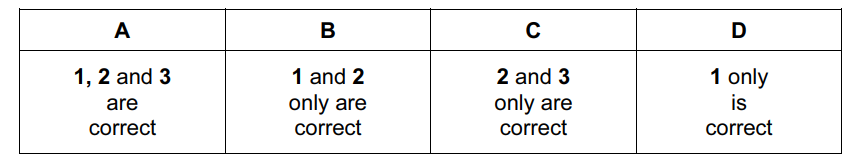
Answer/Explanation
Ans:
D
Question
But-2-ene-1,4-diol is converted in two steps through an intermediate X into ketobutanedioic acid.
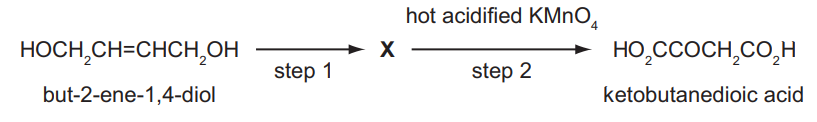
What could be the reagent for step 1 and the intermediate X?
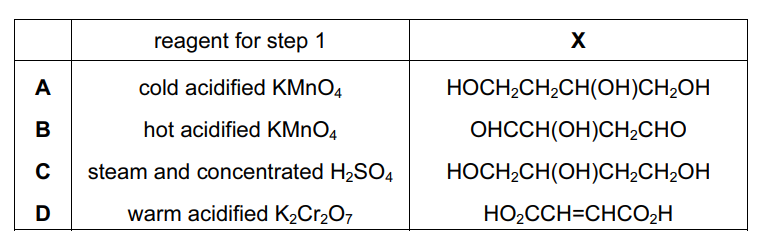
Answer/Explanation
Ans:
C
Question
Compound C is used in textile and leather processing.
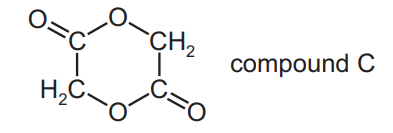
Which starting material(s), on gentle heating with a few drops of concentrated sulfuric acid, generates compound C?
- CH3COOH only
- HOCH2COOH only
- CH3COOCH2COOH only
- CH3COOH mixed with HOCH2COOH
Answer/Explanation
Ans:
B
Question
Fumaric acid can be converted into oxaloacetic acid by a two-step process involving the intermediate Q.
\(HO_{2}CCH=CHCO_{2}H\xrightarrow[]{step1}Q\xrightarrow[]{step2}HO_{2}CCOCH_{2}CO_{2}H\)
fumaric acid oxaloacetic acid
Each of these steps can be achieved in the laboratory by a single reagent.
What could be the intermediate Q and the reagent for step 2?
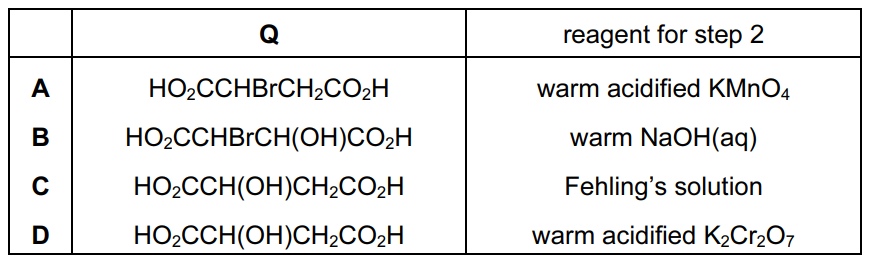
Answer/Explanation
Ans:
D