Question
A series of reactions based on propanoic acid is shown
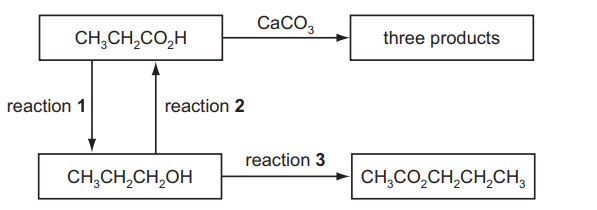
(a) Write an equation for reaction $\mathbf{1}$, using $[\mathrm{H}]$ to represent the reducing agent.[2]
(b) (i) What type of reaction is reaction 2?
(ii) Suggest a suitable reagent and conditions for reaction 2.[2]
(c) Write an equation for the reaction of propanoic acid with calcium carbonate, $\mathrm{CaCO}_3$.[2]
(d) (i) Suggest a suitable reagent and conditions for reaction 3.[2]
(ii) Identify the other product of reaction 3 .[1][Total: 10]
▶️Answer/Explanation
Ans:
(a) $\quad \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}+4[\mathrm{H}] \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}+\mathrm{H}_2 \mathrm{O}$
(b) (i) Oxidation
(ii) Sodium/potassium dichromate or correct formula $\mathrm{H}^{+} /$acidified and (heat under) reflux
(c) $\quad 2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}+\mathrm{CaCO}_3 \rightarrow\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2\right)_2 \mathrm{Ca}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
(d) (i) $\mathrm{CH}_3 \mathrm{CO}_2 \mathrm{H}$
warm/hot/high temperature/heat/reflux AND concentrated sulfuric acid
(ii) water (or hydrogen chloride or ethanoic acid)
Question
A reaction sequence is shown.
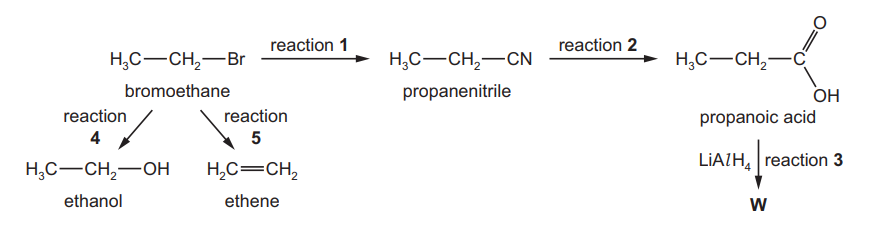
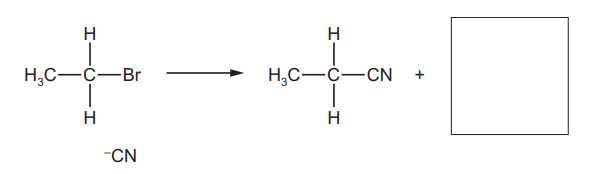
(a) Complete the diagram to show the mechanism of reaction 1. Include all necessary charges, partial charges, lone pairs and curly arrows.
(b) (i) Give the name of the type of reaction involved in reaction 3.
……………………………………………………………………………………………………………………… [1]
The infra-red spectrum of the propanoic acid produced by reaction 2 is shown.
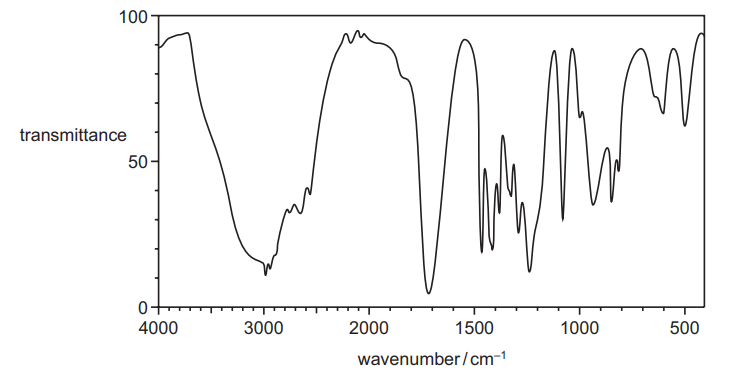
(ii) Describe and explain the main difference between the infra-red spectrum of W and that of propanoic acid………………………………………………………………………………………………………………………. [2]
(c) (i) Reactions 4 and 5 use the same reagent.
Give the reagent and conditions needed for reaction 4.
reagent
conditions[2]
(ii) Give the conditions needed for reaction 5.[1]
(d) Under appropriate conditions, ethanol and propanoic acid undergo a condensation reaction.
(i) State the condition necessary for the reaction.[1]
(ii) Draw the skeletal formula of the organic product of this reaction.[1]
(iii) Name the organic product of this reaction.[1]
(e) V reacts with acidified manganate(VII) ions in two different ways depending on the conditions, as shown in the reaction sequence below
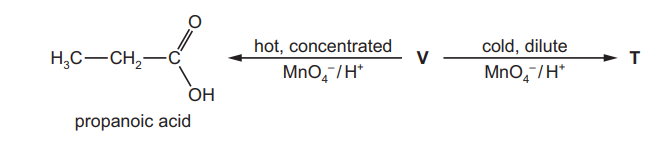 \
\
$\mathbf{V}$ decolourises bromine water.
When the acidified manganate(VII) is hot and concentrated, propanoic acid is the only organic product.
When the acidified manganate(VII) is cold and dilute, the organic product is $\mathbf{T}$ which has two chiral centres.
(i) Give the structural formulae of $\mathbf{V}$ and $\mathbf{T}$.
V…………………………………………….. T………………………………….[2]
(ii) Identify the types of stereoisomerism shown by $\mathbf{V}$ and $\mathbf{T}$.
V …………………………………………….T…………………………………………..[2][Total: 15]
▶️Answer/Explanation
Ans:
5 (a) 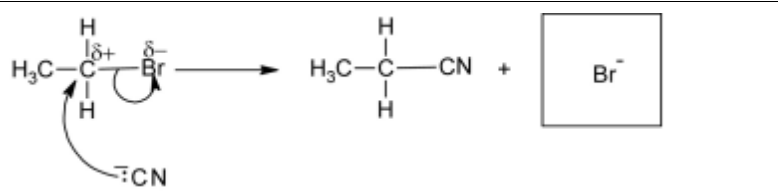
(b) (i) reduction
(ii) disappearance of peak / dip/ trough/ absorption at 1680–1730
due to (loss of) C=O
OR
peak at 3200–3650
due to (alcohol) O—H (formation)
(c) (i) sodium/potassium hydroxide
aqueous
(ii) ethanol
(d) (i) (conc) $\mathrm{H}^{+} /$(conc) acid $/$(conc) $\mathrm{H}_2 \mathrm{SO}_4 /$ (conc) $\mathrm{H}_3 \mathrm{PO}_4$
(ii)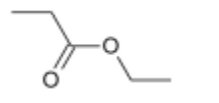
(e) (i) $\quad \begin{aligned} & \mathbf{v}=\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHCHCH} \mathrm{CH}_3 / \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}=\mathrm{CHCH}_2 \mathrm{CH}_3 \\ & \mathbf{T}=\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}(\mathrm{OH}) \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_2 \mathrm{CH}_3\end{aligned}$
(ii) V = geometric(al)/ cis-trans /E–Z
T = optical