Question:
Ethanedioic acid has the formula \(HO_{2}CCO_{2}H\). What is the formula of aluminium ethanedioate?
A \(A \imath C_{2}O_{4}\)
B \(A \imath (C_{2}O_{4})_{3}\)
C \(A \imath _{2}C_{2}O_{4}\)
D \(A\imath_{2}(C_{2}O_{4})_{3}\)
▶️Answer/Explanation
Ans:D
Question:
An organic compound, $\mathrm{T}$, does not fizz when aqueous sodium carbonate is added to it.
Compound $\mathrm{T}$ contains $27.6 \%$ by mass of oxygen.
What could be the identity of T?
1. propanal
2. ethyl butanoate
3. 3-methylpentanoic acid
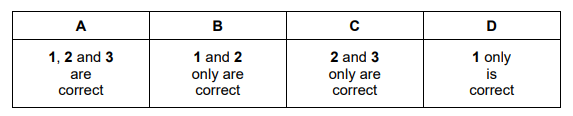
▶️Answer/Explanation
Ans:B
Question
Which two compounds can react together to produce an ester?
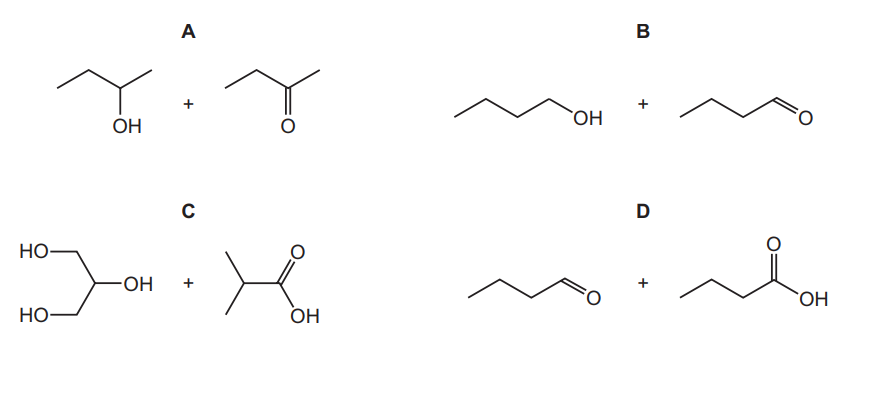
▶️Answer/Explanation
Ans:C
Question
Which row of the table is correct?
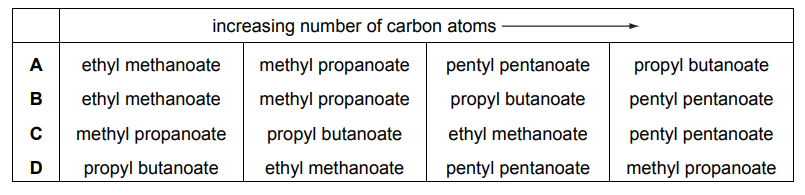
▶️Answer/Explanation
Ans:B
Question
Which mixture could be used to produce propyl methanoate?
A $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$ and $\mathrm{CH}_3 \mathrm{OH}$
B $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}$ and $\mathrm{HCO}_2 \mathrm{H}$
C $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}$ and $\mathrm{HCO}_2 \mathrm{H}$
D $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$ and $\mathrm{CH}_3 \mathrm{OH}$
▶️Answer/Explanation
Ans:C
Question
The fragrance compounds of perfumes are often dissolved in solvent $\mathbf{Y}$, which has a molecular formula $\mathrm{C}_7 \mathrm{H}_{12} \mathrm{O}_4$. It is made by reacting propane-1,2-diol with ethanoic acid in the presence of an acid catalyst.
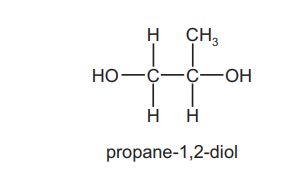
What is the structure of solvent Y?
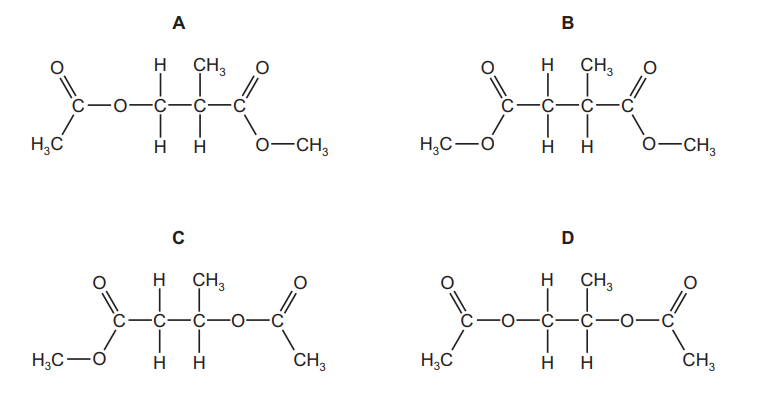
▶️Answer/Explanation
Ans:D
Question
Compound X contains a single ester group. X contains 27.6% by mass of oxygen.
Which pair of products could be produced by the hydrolysis of X?
A butan-1-ol and ethanoic acid
B ethanol and propanoic acid
C methanol and methanoic acid
D propan-2-ol and butanoic acid
Answer/Explanation
Ans: A
Question
How many esters have the molecular formula \(C_4H_8O_2\)?
A 2 B 3 C 4 D 5
Answer/Explanation
Ans: C
Question
The compound cetyl palmitate, C15H31CO2C16H 33, is a waxy solid.
Cetyl palmitate is heated under reflux with an excess of aqueous sodium hydroxide.
Which products will be formed?
A C15H31ONa and C16H33CO2Na
B C15H31CO2Na and C16H33ONa
C C15H31OH and C16H33CO2Na
D C15H31CO2Na and C16H 33OH
Answer/Explanation
Answer: D
Question
The structural formula of an ester is (CH3)2CHOCO(CH2) 2CH3.
This ester is boiled with aqueous hydrochloric acid.
Which two products are formed?
A propan-1-ol and butanoic acid
B propan-2-ol and butanoic acid
C propan-1-ol and propanoic acid
D propan-2-ol and propanoic acid
Answer/Explanation
Answer B
Question
When CH2(OH)CH=CHCO2H is warmed with a little concentrated sulfuric acid, a cyclic compound is formed.
What is the skeletal formula of the cyclic compound?
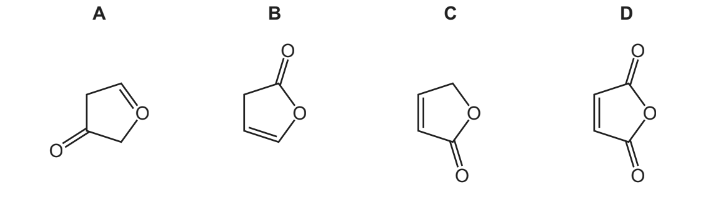
▶️Answer/Explanation
Answer C
Question
Which compounds produce three different organic products when hydrolysed?
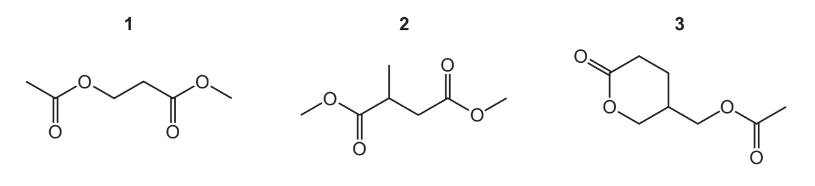
▶️Answer/Explanation
Answer D
Question
The diester shown can be hydrolysed by heating with an excess of aqueous sodium hydroxide.
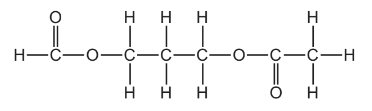
What would the products of this reaction be?
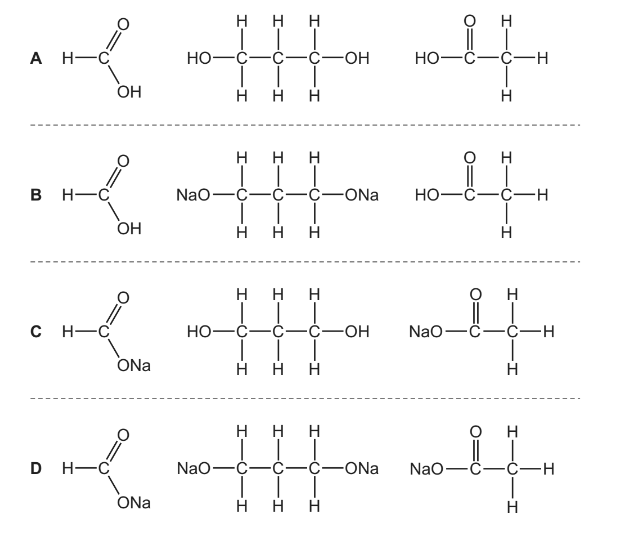
Answer/Explanation
Answer C
Question
Ethyl propanoate is refluxed with aqueous sodium hydroxide. The alcohol produced is then reacted with methyl propanoic acid to make a second ester.
What is the structural formula of this second ester?
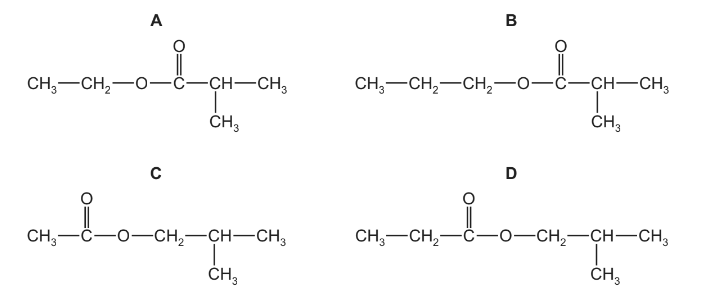
Answer/Explanation
Answer A
Question
Hydrogen ions catalyse the hydrolysis of esters.
Which statement is correct?
A The hydrogen ions act as a heterogeneous catalyst.
B The hydrogen ions are in the same phase as the reactants.
C The hydrogen ions are used up in the reaction.
D The hydrogen ions have no effect on the activation energy of the reaction.
Answer/Explanation
Answer B
Question
Ethanedioate ions, \(C_{2}O_{4}^{2-}\), react with a suitable reagent to form CO2. A half-equation for this reaction is shown.
\(C_{2}O_{4}^{2-}\rightarrow 2CO_{2}+2e^{-}\)
Which row is correct?
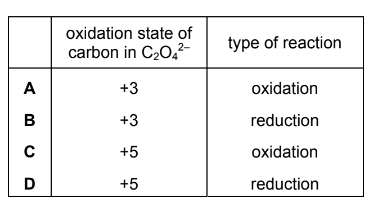
▶️Answer/Explanation
Answer A
Question
Ethanedioic acid has the formula HO$_2$CCO$_2$H.
What is the formula of aluminium ethanedioate?
A AlC2O4 B Al(C2O4)3 C Al2C2O4 D Al2(C2O4)3
Answer/Explanation
Answer:
D
Question
One molecule of ammonia reacts with one molecule of ethyl methanoate, HCO2C2H5, to produce one molecule of methanamide, HCONH2, and only one other molecule, X.
One molecule of methanamide decomposes on heating strongly to produce one molecule of ammonia and only one other molecule, Y.
What could be the identities of X and Y?
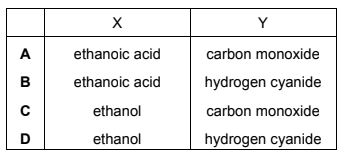
Answer/Explanation
Answer:
C
Question
A sample of the ester \(CH_3CH_2CH_2CO_2CH_2CH_3\) is hydrolysed. The product mixture is then treated with hot, acidified \(KMnO_4\).
What are the final carbon-containing products?
A \(CH_3CH_2CO_2H\) only
B \(CH_3CO_2H + CH_3CH_2CO_2H\)
C \(CH_3CO_2H + CH_3CH_2CH_2CO_2H\)
D \(CH_3CH_2OH + CH_3CH_2CH_2CO_2H\)
Answer/Explanation
Ans: C
Question
Lactide is an intermediate in the manufacture of a synthetic fibre.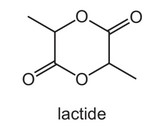
Which compound, on heating with an acid catalyst, can produce lactide?
A hydroxyethanoic acid
B 2-hydroxybutanoic acid
C 2-hydroxypropanoic acid
D 3-hydroxypropanoic acid
Answer/Explanation
Ans: C
Question
Which formula represents an ester that will form propanoic acid on hydrolysis with dilute sulfuric acid?
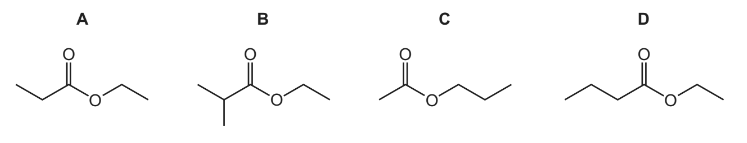
Answer/Explanation
Answer: A
Question
Part of the structure of a fungicide, strobilurin, is shown. R and R’ are inert groups.
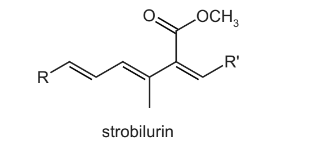
In this reaction, strobilurin is warmed with aqueous sulfuric acid producing compound X. Compound X is then treated with hydrogen in the presence of a nickel catalyst producing compound Y.
What could be the structure of compound Y?
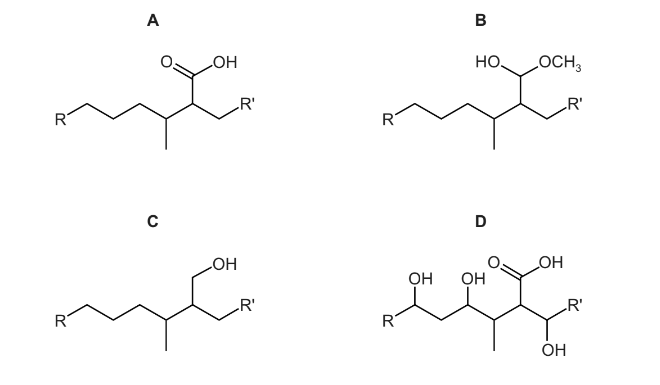
Answer/Explanation
Answer: A
Question
D potassium cyanide in ethanol
Answer/Explanation
Answer: D
Question
The ester CH3CH2CH2CO2CH2CH(CH3)2 was hydrolysed under acidic conditions.
What are the organic products of this hydrolysis?
A butanoic acid and 2-methylpropan-1-ol
B butanoic acid and 2-methylpropan-2-ol
C butan-1-ol and 2-methylpropanoic acid
D propanoic acid and 2-methylpropan-1-ol
Answer/Explanation
Answer: A
Question
Cyclic esters are also known as lactones. Delta lactone is used as a solvent and in the manufacture of polyesters.
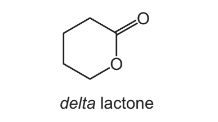
From which compound could delta lactone be made by a single reaction?
A HOCH2CH2CH2CH2CHO
B HOCH2CH2CH2CH2CO2H
C HOCH2CH2CH2CH2CH2OH
D HOCH2CH2CH2CH2CH2CO2H
Answer/Explanation
Answer: B
Question
The structural formula of compound X is shown below.
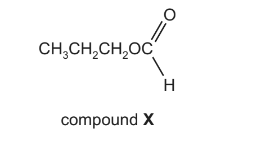
What is the name of compound X and how does its boiling point compare with that of butanoic
acid?
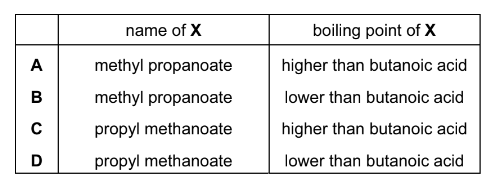
Answer/Explanation
Answer: D
Question
How many isomeric esters have the molecular formula \(C_{4}H_{8}O_{2} \)?
A 2 B 3 C 4 D 5
Answer/Explanation
Ans:C
Question
The ester \(CH_{3}CH_{2}CH_{2}CO_{2]CH_{3}\) is responsible for the aroma of apples.
When this ester is hydrolysed by acid in the stomach, what is the empirical formula of the organic acid produced?
A \( CH_{2}O \) B \(CH_{4}O\) C \(C_{2}H_{4}O\) D \( C_{3}H6O_{2}\)
Answer/Explanation
Ans:C
Question
Methyl methyl-propenoate is the monomer used to make Perspex. Which diagram correctly shows methyl methyl-propenoate?
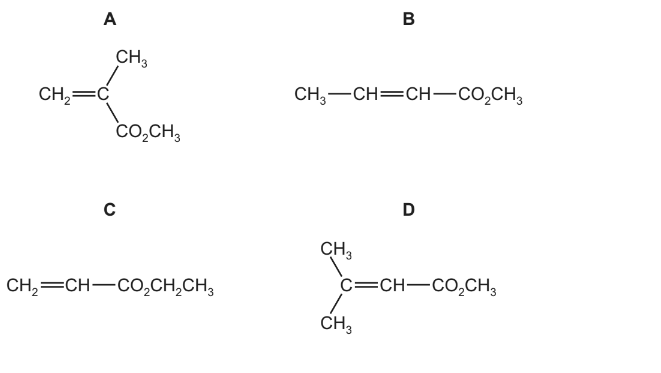
Answer/Explanation
Ans:A
Question
The molecule responsible for the pineapple flavour used in sweets is \(CH_{3}CH_{2}CH_{2}CO_{2}CH_{2}CH_{3}\)
Which statements about this molecule are correct?
1 The name of this compound is ethyl butanoate.
2 This compound is a structural isomer of hexanoic acid.
3 When this compound is heated with aqueous sodium hydroxide, the products are butan-1-ol and sodium ethanoate.
The responses A to D should be selected on the basis of

Answer/Explanation
Ans:B
Question
An ester with an odour of banana has the following formula.
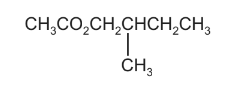
Which pair of reactants, under suitable conditions, will produce this ester?
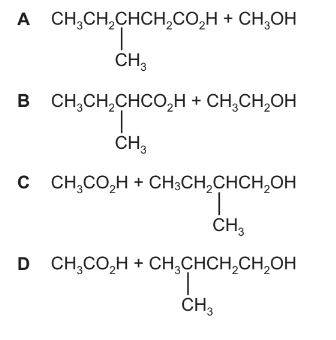
Answer/Explanation
Ans:C
Question
An experiment is set up to measure the rate of hydrolysis of ethyl ethanoate.
\(CH_3CO_2C_2H_5 + H_2O \leftrightarrow CH_3CO_2H + C_2H_5OH\)
The hydrolysis is found to be slow in neutral aqueous solution but it proceeds at a measurable rate when the solution is acidified with hydrochloric acid.
What is the function of the hydrochloric acid?
A to dissolve the ethyl ethanoate
B to ensure that the reaction reaches equilibrium
C to increase the reaction rate by catalytic action
D to suppress ionisation of the ethanoic acid formed
Answer/Explanation
Ans: C
Question
Use of the Data Booklet is relevant to this question.
A sample of ethyl propanoate is hydrolysed by heating under reflux with aqueous sodium hydroxide. The two organic products of the hydrolysis are separated, purified and weighed.
Out of the total mass of products obtained, what is the percentage by mass of each product?
- 32.4% and 67.6%
- 38.3% and 61.7%
- 42.3% and 57.7%
- 50.0% and 50.0%
Answer/Explanation
Ans:
A