Question
Methylamine, $\mathrm{CH}_3 \mathrm{NH}_2$, has very similar chemical properties to ammonia, $\mathrm{NH}_3$.
Methylamine reacts with hydrogen chloride to form a white crystalline salt, methylammonium chloride.
$
\mathrm{CH}_3 \mathrm{NH}_2+\mathrm{HCl} \rightarrow \mathrm{CH}_3 \mathrm{NH}_3{ }^{+} \mathrm{Cl} l^{-}
$
A sample of methylammonium chloride is heated with aqueous sodium hydroxide.
What are the products?
A ammonia, sodium chloride and water
B ammonia, sodium hydrogencarbonate and sodium chloride
C methylamine, hydrogen chloride and water
D methylamine, sodium chloride and water
▶️Answer/Explanation
Ans:D
Question
Coniine is the major constituent of the poison ‘oil of hemlock’.
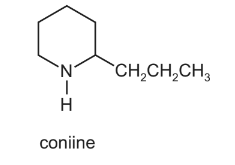
Coniine can be synthesised by reacting ammonia with a dibromo compound, X.
\[
\begin{gathered}
\mathrm{NH}_3+\mathrm{C}_8 \mathrm{H}_{16} \mathrm{Br}_2 \rightarrow \text { coniine }+2 \mathrm{HBr} \\
\mathbf{X}
\end{gathered}
\]
What is the name of compound X?
A 1,1-dibromo-2-propylcyclopentane
B 1,2-dibromo-2-propylcyclopentane
C 1,4-dibromooctane
D 1,5-dibromooctane
Answer/Explanation
Ans:D
Question
How can a good yield of ethylamine be made using bromoethane as starting material?
- by heating bromoethane with an excess of ammonia gas in a sealed tube
- by adding dilute aqueous ammonia to bromoethane at room temperature
- by heating bromoethane under reflux with aqueous ammonium chloride
Answer/Explanation
Ans:
D