Question
Alkanes and alkenes both react with bromine.
(a) Explain how and why bromine can be used to distinguish between an alkene and an alkane.
(b) The reaction of ethane with bromine forms a mixture of products.
(i) State the essential conditions for this reaction to occur.
(ii) Give the full name of the mechanism of this reaction.
(iii) Give the equation for a termination step that could occur, producing a hydrocarbon.
(iv) Give the equation for one propagation step involved in the formation of dibromoethane
from bromoethane during this reaction.
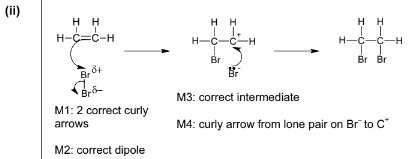
(c) The reaction of ethene with bromine forms a single product.
(i) Give the full name of the mechanism of this reaction.
(ii) Complete the diagram below to illustrate this mechanism. Include all relevant charges, partial charges, curly arrows and lone pairs.
![]()
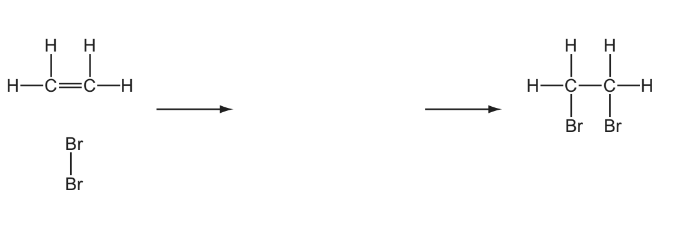
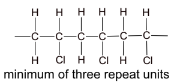
(d) Chloroethene can be polymerised to form a polymer commonly known as PVC. Draw a diagram of the structure of PVC including three repeat units.
(e) Chloroethane undergoes a series of reactions as shown in the diagram below.
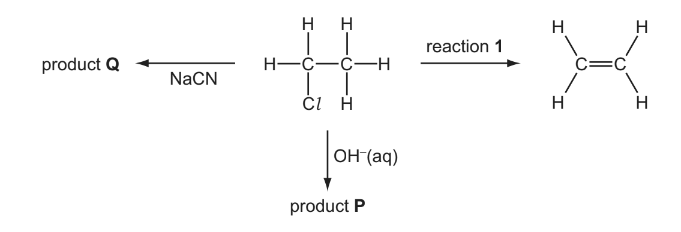
(i) Give the reagent and conditions necessary for reaction 1.
(ii) Give the skeletal formula of product P.
(iii) Give the displayed formula and the name of product Q.
Answer/Explanation
(a) decolourisation with an alkene at room conditions / quickly / easily /
OR alkane needs higher temp/UV/ is slow at room conditions double/ π /pi bond/C =C present in alkenes
(b) (i) UV light/ sunlight/ high temperature
(ii) (Free) radical
Substitution
(iii) \(•C_{2}H_{5} + •C_{2}H_{5} \rightarrow C_{4}H_{10}\)
(iv) \(C_{2}H_{5}Br + Br^{•}\rightarrow •C_{2}H_{4}Br + HBr\) OR
\(•C2H4Br + Br_{2} \rightarrow C_{2}H_{4}Br_{2} + Br^{•}\)
(c) (i) Electrophilic
Addition
(d)
(e) (i) NaOH/KOH
ethanolic / alcoholic AND heat/reflux
(ii)![]()
(iii) 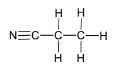
Propanenitrile/propanonitrile/ propionitrile/ ethyl cyanide/ cyanoethane
Question
(a) A series of reactions starting from 1-bromobutane is shown.
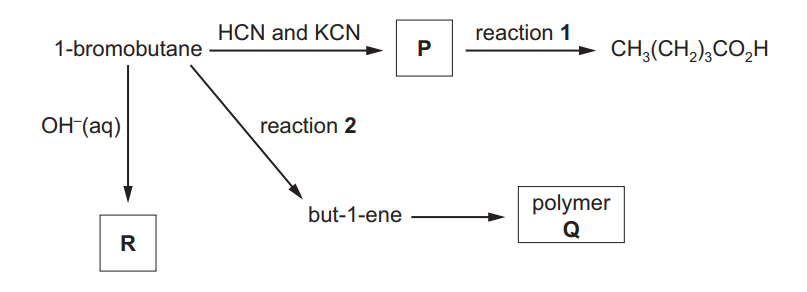
(i) Draw the displayed formula of compound P
(ii) Identify the reagent(s) and conditions for reactions 1 and 2.
reaction 1 …………………………………………………………………………………………………………….
reaction 2 ……………………………………………………………………………………………………………. [2]
(iii) Draw the structure of the repeat unit of polymer Q.
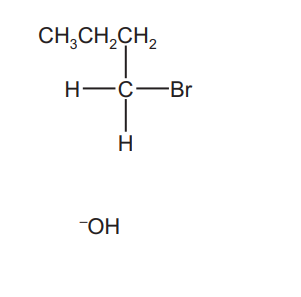
(b) Complete the reaction scheme to show the mechanism of the reaction of 1-bromobutane with $\mathrm{OH}^{-}(\mathrm{aq})$ to produce $\mathbf{R}$.
Include all necessary charges, dipoles, lone pairs and curly arrows and the structure of $\mathbf{R}$.
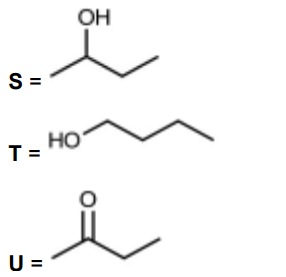
(c) But-1-ene reacts with steam as shown to form a mixture of two structural isomers, S and T.
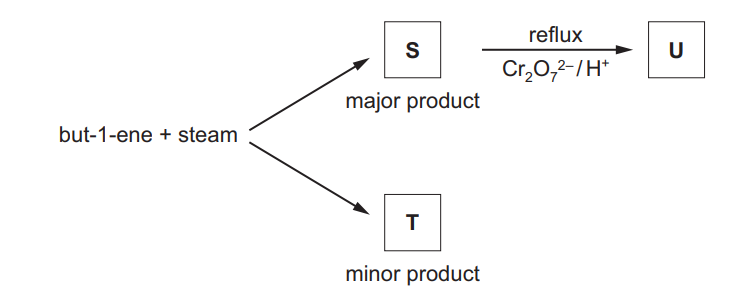
S can be oxidised with acidified potassium dichromate(VI) to form compound U.
S and U both react with alkaline aqueous iodine.
(i) Identify the type of reaction that occurs when but-1-ene reacts with steam.
……………………………………………………………………………………………………………………… [1]
(ii) State what can be deduced about the structure of S from its reaction with alkaline aqueous iodine.
……………………………………………………………………………………………………………………… [1]
(iii) Explain why S is the major product of the reaction of but-1-ene with steam
(iv) Draw the skeletal formulae of S, T and U
 [3]
[3]
(v) Write an equation to represent the oxidation of S to U by acidified potassium dichromate(VI).
You should use [O] to represent the oxidising agent.[1]
(d) $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$ is a colourless liquid with an unpleasant odour.
It reacts with methanol in the presence of an acid catalyst to produce an organic product $\mathbf{V}$, which has a pleasant fruity smell.
(i) Name V. [1]
(ii) A student analysed $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{CO}_2 \mathrm{H}$, methanol and $\mathbf{V}$ using infra-red spectroscopy. The spectra were returned to the student without labels.
Identify which of the infra-red spectra, $\mathrm{X}, \mathrm{Y}$ or $\mathrm{Z}$, corresponds to $\mathbf{V}$.
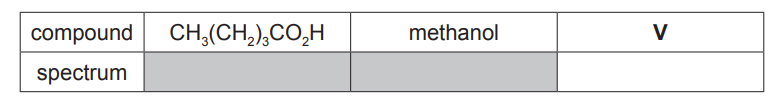
Explain your answer with reference to relevant features of the three spectra in the region above $1500 \mathrm{~cm}^{-1}$. [4]
▶️Answer/Explanation
Ans:
(a)(i)
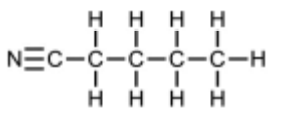
(a)(ii) reaction 1 = HCl(aq)
reaction 2 = (conc.) NaOH/KOH AND ethanol
(a)(iii)
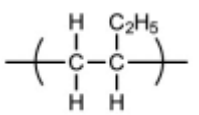
C–C backbone with dangling bonds
rest of structure
(b)
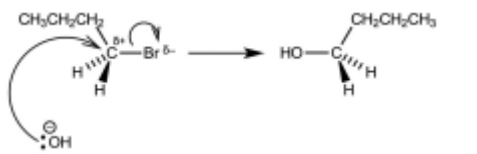
lone pair on O AND curly arrow from O to C of C–Br
dipole on C–Br AND curly arrow from C–Br to Br
product (butan-1-ol)
(c)(i) (electrophilic) addition
(c)(ii) $\quad \mathbf{S}$ has $\mathrm{CH}_3 \mathrm{CHOH}$ OR methyl/ $\mathrm{CH}_3$ group next to $\mathrm{CHOH}$
(c)(iii) positive inductive effect of more alkyl groups /more alkyl groups donate electron density
secondary carbocation / secondary intermediate is more stable (than primary)
(c)(iv)
$(c)(v) \quad \mathrm{CH}_3 \mathrm{CHOHCH}_2 \mathrm{CH}_3+[\mathrm{O}] \rightarrow \mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{CH}_3+\mathrm{H}_2 \mathrm{O}$
(d)(i) methyl pentanoate
(d)(ii) (compound V is) spectrum X
spectra $X$ and $Z$ show a $C=O$ (stretch) at $1730\left(\mathrm{~cm}^{-1}\right)$
spectra $Y$ and $Z$ show O-H (stretches) above $2500\left(\mathrm{~cm}^{-1}\right)$
$\mathbf{V}$ has a $\mathrm{C}=\mathrm{O}$ (bond) and no $\mathrm{O}-\mathrm{H}$ (bond)