Question:
Substance Q is a hydrocarbon. When 1.00 g of Q is completely burned, 3.22 g of carbon dioxide is produced. What could be the identity of Q?
A cyclohexene
B cyclopentane
C ethene
D pentane?
▶️Answer/Explanation
Ans:A
Question:
X and Y are oxides of different Period 3 elements. If one mole of X is added to water, the solution formed is neutralised by exactly one mole of Y. What could be the identities of X and Y?
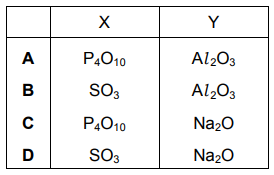
▶️Answer/Explanation
Ans:D
Question
For which hydrocarbon are the molecular and empirical formulae the same?
A butane
B ethane
C pent-1-ene
D propane
▶️Answer/Explanation
ANS:D
Question
Compounds $\mathrm{J}$ and $\mathrm{K}$ each contain $40 \%$ carbon by mass.
What could $\mathrm{J}$ and $\mathrm{K}$ be?
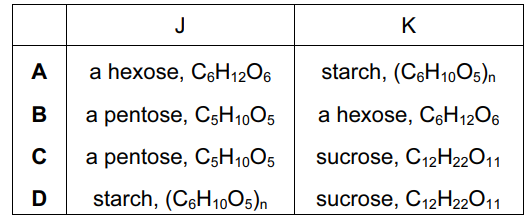
▶️Answer/Explanation
Ans:B
Question
Compound X contains the elements C, H and O only. 2.00 g of X produces 4.00 g of carbon dioxide and 1.63 g of water when completely combusted.
What is the empirical formula of X?
A \(CHO_2\) B \(C_2H_2O\) C \(C_2H_4O\) D \(CH_2O_2\)
Answer/Explanation
Ans: C
Question
A student reacts 1 mol of copper with concentrated nitric acid to produce 1 mol of copper(II) nitrate, 2 mol of water and substance X. No other product is formed. Substance X does not contain copper or hydrogen.
What could be substance X?
A \(N_2\) B \(N_2O\) C NO D \(NO_2\)
Answer/Explanation
Ans: D
Question
2.0 g of ammonium nitrate, NH4NO3, decomposes to give 0.90 g of water and a single gas.
What is the identity of the gas?
A NO B NO2 C N2O D N2
Answer/Explanation
Answer C
Question
Four elements, W, X, Y and Z, have electronic configurations as shown.
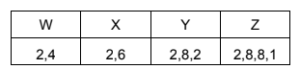
Which formulae represent compounds that have boiling points below room temperature?
1 WX2
2 YX
3 Z2X
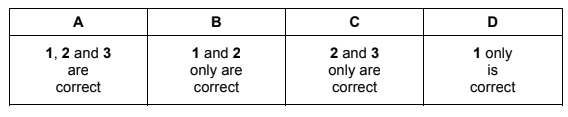
Answer/Explanation
Answer D
Question
Sodium reacts with 1 mol of compound Y to produce 1 mol of H2(g).
Which compound could Y be?
A CH3CH2CH2CH2OH
B (CH3)3COH
C CH3CH2CH2CO2H
D CH3CH(OH)CO2H
Answer/Explanation
Answer D
Question
An organic molecule W contains 3 carbon atoms. It requires 4.5 molecules of oxygen for complete combustion.
What could W be?
A propane
B propanoic acid
C propanone
D propan-1-ol
Answer/Explanation
Answer D
Question
The Mr of compound X is 72. The composition by mass of X is 66.7% carbon, 11.1% hydrogen and 22.2% oxygen. X gives an orange precipitate with 2,4-dinitrophenylhydrazine reagent. X does not react with Fehling’s reagent.
What can be deduced from this information?
1 X is a carbonyl compound.
2 X is a ketone.
3 X is butanone.

Answer/Explanation
Answer A
Question
Compound Q contains 40% carbon by mass.
What could Q be?
1 glucose, C6H12O6
2 starch, (C6H10O5)n
3 sucrose, C12H22O11
The responses A to D should be selected on the basis of
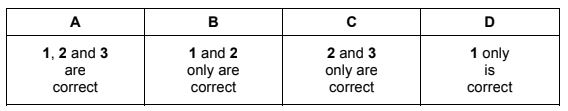
Answer/Explanation
Answer:
D
Question
71.0 g of chlorine, \(Cl_2\), react with an excess of sodium hydroxide solution at a particular
temperature. The reaction produces exactly 35.5 g of product X.
What is product X?
A \(H_2O\) B NaCl C NaClO D \(NaClO_3\)
Answer/Explanation
Ans: D
Question
The elements Cl , Mg, Si and S are all in Period 3.
What is the correct sequence of the melting points of these elements, from lowest to highest?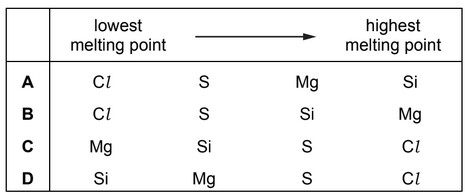
Answer/Explanation
Ans: A
Question
The complete combustion of 2 moles of a straight chain alkane produces 400 \(dm^3\) of carbon dioxide measured at 301K and 1 × \(10^5\)Pa. Carbon dioxide can be assumed to behave as an ideal gas under these conditions.
What is the formula of the straight chain alkane?
A \(C_8H_{18}\) B \(C_{16}H_{34}\) C \(C_{20}H_{42}\) D \(C_{40}H_{82}\)
Answer/Explanation
Ans: A
Question
Which formula represents the empirical formula of a compound?
A \(C_2H_4O\) B \(C_2H_4O_2\) C \(C_6H_{12}\) D \(H_2O_2\)
Answer/Explanation
Ans: A
Question
1.15 g of a metallic element needs 300 cm3 of oxygen for complete reaction, at 298K and 1 atm pressure, to form an oxide which contains O2– ions.
What could be the identity of this metallic element?
A calcium
B magnesium
C potassium
D sodium
Answer/Explanation
Answer: D
Question
The reaction between acidified dichromate(VI) ions, Cr2O72–, and aqueous Fe2+ ions results in the dichromate(VI) ions being reduced to Cr3+ ions.
What is the correct equation for this reaction?
A \(Cr_{2}O_{7}^{2-} + Fe^{2+} + 14H^{+} \rightarrow 2Cr^{3+} + Fe^{3+} + 7H_{2}O\)
B \(Cr_{2}O_{7}^{2-} + 2Fe^{2+} + 14H^{+} \rightarrow 2Cr^{3+} + 2Fe^{3+} + 7H_{2}O\)
C \(Cr_{2}O_{7}^{2-} + 3Fe^{2+} + 14H^{+} \rightarrow 2Cr^{3+} + 3Fe^{3+} + 7H_{2}O\)
D \(Cr_{2}O_{7}^{2-} + 6Fe^{2+} + 14H^{+} \rightarrow 2Cr^{3+} + 6Fe^{3+} + 7H_{2}O\)
Answer/Explanation
Answer: D
Question
The hydrocarbon C17H36 can be cracked. Which compound is the least likely to be produced in this reaction?
A \(C_{3}H_{8}\)
B \(C_{4}H_{8} \)
C \(C_{8}H_{16} \)
D \(C_{16}H_{34}\)
Answer/Explanation
Ans:D
