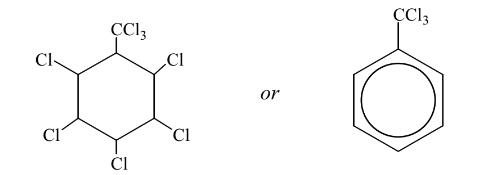Question
(a) One atom of each of the four elements H, C, N and O can bond together in different ways.
Two examples are molecules of cyanic acid, HOCN, and isocyanic acid, HNCO. The atoms are bonded in the order they are written.
(i) Draw ‘dot-and-cross’ diagrams of these two acids, showing outer shell electrons only.
(ii) Suggest the values of the bond angles HNC and NCO in isocyanic acid.
HNC ………………………… NCO …………………………
(iii) Suggest which acid, cyanic or isocyanic, will have the shorter C–N bond length.
Explain your answer.
(b) (i) Isocyanic acid is a weak acid.
\(HNCO \leftrightarrow H^+ + NCO^–\) \(K_a = 1.2 × 10^{–4}\) mol\(dm^{–3}\)
Calculate the pH of a 0.10mol\(dm^{–3}\) solution of isocyanic acid.
pH = ………
(ii) Sodium cyanate, NaNCO, is used in the production of isocyanic acid.
Sodium cyanate is prepared commercially by reacting urea, \((NH_2)_2CO\), with sodium carbonate. Other products in this reaction are carbon dioxide, ammonia and steam.
Write an equation for the production of NaNCO by this method.
(c) Barium hydroxide, \(Ba(OH)_2\), is completely ionised in aqueous solutions.
During the addition of 30.0 \(cm^3\) of 0.100mol\(dm^{–3}\) \(Ba(OH)_2\) to 20.0\(cm^3\) of 0.100mol\(dm^{–3}\) isocyanic acid, the pH was measured.
(i) Calculate the \([OH^–]\) at the end of the addition.
\([OH^–]\) = ………………………… mol\(dm^{–3}\)
(ii) Use your value in (i) to calculate \([H^+]\) and the pH of the solution at the end of the addition.
final \([H^+]\) = ………………………… mol\(dm^{–3}\)
final pH = …………………………
(iii) On the following axes, sketch how the pH changes during the addition of a total of 30.0\(cm^3\) of 0.100mol\(dm^{–3}\) \(Ba(OH)_2\) to 20.0\(cm^3\) of 0.100mol\(dm^{–3}\) isocyanic acid.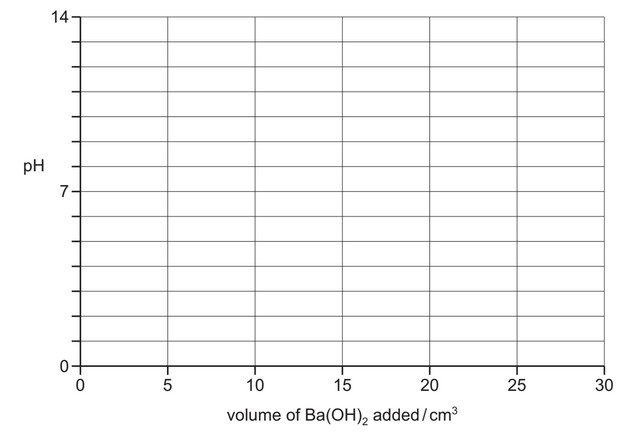
(d) The cyanate ion, \(NCO^–\), can act as a monodentate ligand.
(i) State what is meant by the terms
monodentate, ……………………………………………………………………………………………………….
ligand. …………………………………………………………………………………………………………………
Silver ions, \(Ag^+\), react with cyanate ions to form a linear complex.
(ii) Suggest the formula of this complex, including its charge.
(e) When heated with HCl(aq), organic isocyanates, RNCO, are hydrolysed to the amine salt,
\(RNH_3Cl\), and \(CO_2\).
\(RNCO + H_2O + HCl \rightarrow RNH_3Cl + CO_2\)
A 1.00g sample of an organic isocyanate, RNCO, was treated in this way, and the \(CO_2\) produced
was absorbed in an excess of aqueous \(Ba(OH)_2\) according to the equation shown. The solid
\(BaCO_3\) precipitated weighed 1.66g.
\(Ba(OH)_2(aq) + CO_2(g) \rightarrow BaCO_3(s) + H_2O(l)\)
(i) Calculate the number of moles of \(BaCO_3\) produced.
moles of \(BaCO_3\) = …………………………
(ii) Hence calculate the Mr of the organic isocyanate RNCO.
\(M_r\) of RNCO = …………………………
The R group in RNCO and \(RNH_3Cl\) contains carbon and hydrogen only.
(iii) Use your \(M_r\) value calculated in (ii) to suggest the molecular formula of the organic isocyanate RNCO.
molecular formula of RNCO ……………………………………………………………………………..
(iv) Suggest a possible structure of the amine \(RNH_2\), which forms the amine salt, \(RNH_3Cl\).
Answer/Explanation
Answer:
(a) (i) 
16 electrons on each diagram
(ii) HNC = 115–125° AND NCO = 180°
(iii) cyanic acid, because it’s a stronger / higher bond enthalpy / triple / C≡N / more electrons involved bond
(b) (i) \([H^+] = √([HNCO]K_a) = √(0.1 × 1.2 × 10^{–4})\) or 3.46 × \(10^{–3}\)
pH = log [\(H^+\)] = 2.5 (2.46)
(ii) \(Na_2CO_3 + 2(NH_2)_2CO → 2NaNCO + CO_2 + 2NH_3 + H_2O\)
(c) (i) \((n(OH^–)\) at start = (2 × 0.1 × 30) / 1000 = 6 × \(10^{–3}\) mol)
\((n(OH^–)\) reacted = (0.1 × 20) / 1000 = 2 × \(10^{–3}\) mol)
\(n(OH^–)\) remaining = (6–2) × \(10^{–3}\) = 4 × \(10^{–3}\) mol, (in 50 \(cm^3\))
so \([OH^–]_{end}\) = (4 × \(10^{–3}\) × 1000) / 50 = 0.08 mol \(dm^{–3}\)
(ii) \([H^+] = K_w / [OH^–] = (1 × 10^{–14}) / 0.08 = 1.25 × 10^{–13}\) mol \(dm^{–3}\)
so pH = –log(1.25 × \(10^{–13}\)) = 12.9
(iii) curve starts at 2.46 / 2.5
vertical portion (end point) at vol added = 10.0 \(cm^3\)
finishes at pH = 12.9
(d) (i) monodentate: (a species that) forms one dative / coordinate bond
ligand: a species that uses a lone pair of electrons to form a dative / coordinate bond to a metal atom / metal ion
(ii) \([Ag(NCO)_2]^–\) or \([Ag(OCN)_2]^–\) correct formula
correct charge
(e) (i) \(n(BaCO_3)\) =1.66 / 197.3 = 8.4(1) × \(10^{–3}\) mol
(ii) n(RNCO) = 8.41 × \(10^{–3}\) mol, so \(M_r\) = 1 / (8.41 × \(10^{–3}\)) = 119
(iii) molecular formula = \(C_7H_5NO\)
(iv) 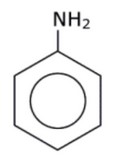
Question
(a) Complete the diagrams to show the energies of the electrons in a carbon atom, a C+ ion and a C– ion.
(b) One of the simple molecular allotropes of carbon is buckminsterfullerene, C60.
(i) What is the hybridisation of the carbon atoms in C60?
(ii) C60 reacts with an excess of hydrogen to form a single product, C60Hx.
Using your answer to (i), suggest a suitable value for x.
(c) Methylbenzene can undergo different reactions to form the products shown below.
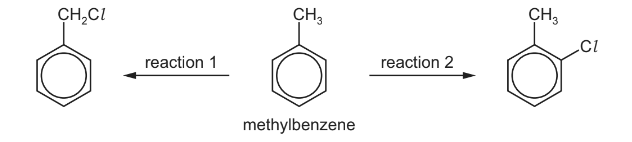
(i) Give the reagents and conditions for these two reactions.
(ii) Name the mechanism of reaction 1.
(iii) Draw the structure of the product obtained if reaction 1 is carried out using an excess of
chlorine.
Answer/Explanation
Answer: (a) 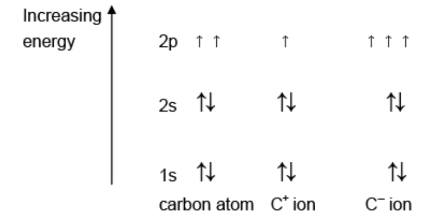
(b) (i) sp2
(b) (ii) x = 60/C60H60
(c) (i) reaction 1: Cl2 and UV light;
reaction 2: AlCl3, Cl2 (NOT aqueous);
c(ii) (free) radical substitution
c(iii)