Question
Nitrobenzene, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2$, can be reduced to phenylamine, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2$, in acid solution in a two step process.
(a) (i) Balance the half-equation for this reaction to work out how many moles of electrons are needed to reduce one mole of nitrobenzene.
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2+\ldots \ldots \ldots \mathrm{e}^{-}+\ldots \ldots \ldots . . \mathrm{H}^{+} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\ldots \ldots \ldots \ldots \mathrm{H}_2 \mathrm{O}$
(ii) The reducing agent normally used is granulated tin and concentrated hydrochloric acid. In the first step, the reduction of nitrobenzene to phenylammonium chloride can be represented by the equation shown.
Use oxidation numbers or electrons transferred to balance this equation. You might find your answer to (i) useful.
$
. \mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2+\ldots . . \mathrm{HCl}+\ldots \ldots . \mathrm{Sn} \rightarrow \ldots \ldots \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_3 \mathrm{Cl}+\ldots \ldots . \mathrm{SnCl}_4+\ldots \ldots . \mathrm{H}_2 \mathrm{O}
$
(b) When $5.0 \mathrm{~g}$ of nitrobenzene was reduced in this reaction, $4.2 \mathrm{~g}$ of phenylammonium chloride, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_3 \mathrm{Cl}$, was produced.
Calculate the percentage yield.
percentage yield of phenylammonium chloride = ……………………….. % [2]
(c) Following the reaction in (b), an excess of $\mathrm{NaOH}(\mathrm{aq})$ was added to liberate phenylamine from phenylammonium chloride.
(i) Calculate the mass of phenylamine, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2$, produced when $4.20 \mathrm{~g}$ of phenylammonium chloride reacts with an excess of $\mathrm{NaOH}(\mathrm{aq})$.
mass of phenylamine = ……………………….. g [1]
The final volume of the alkaline solution of phenylamine in (i) was $25.0 \mathrm{~cm}^3$. The phenylamine was extracted by addition of $50 \mathrm{~cm}^3$ of dichloromethane. After the extraction, the dichloromethane layer contained $2.68 \mathrm{~g}$ of phenylamine.
(ii) Use the data to calculate the partition coefficient, $K_{\text {partition }}$, of phenylamine between dichloromethane and water.
$
K_{\text {partition }}=
$
d) How does the basicity of phenylamine compare to that of ethylamine? Explain your answer.
(e) Phenol can be synthesised from phenylamine in two steps
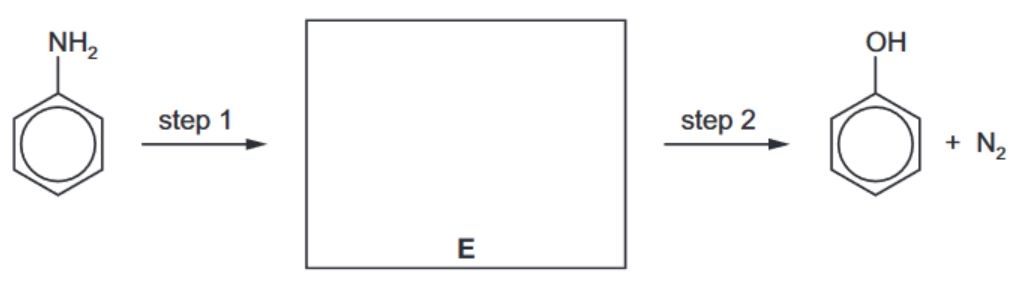
i) State the reagents and conditions for steps 1 and 2.
step 1 ………………………………………………………………………………………………………………….
step 2 …………………………………………………………………………………………………………………. [2]
(ii) Draw the structure of the intermediate compound E in the box above. [1] [Total: 13]
▶️Answer/Explanation
Ans:
(a) (i) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2+6 \mathrm{e}^{-}+6 \mathrm{H}^{+} \longrightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+2 \mathrm{H}_2 \mathrm{O}$
(b) $\quad\left(\mathrm{M}_{\mathrm{r}}\right.$ values: $\left.\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2=123 \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_3 \mathrm{Cl}=129.5\right)$ theoretical yield $=5.0 \times 129.5 / 123=5.26 \mathrm{~g}$ percentage yield $=100 \times 4.2 / 5.26=79.8 \%(80 \%)$
(c) (i) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2=93$
yield of phenylamine $=4.2 \times 93 / 129.5=3.016 \mathrm{~g}$
(ii) mass left in water $=3.016-2.68=0.336 \mathrm{~g}$ $K_{\text {part }}=(2.68 / 50) /(0.336 / 25)=3.99$
(d) phenylamine is less basic that ethylamine the lone pair on $\mathrm{N}$ is delocalised over the ring…
…making it less available for reaction with a proton $/ \delta+\mathrm{H}$
(e) (i) step 1: $\mathrm{HNO}_2 \mathrm{OR}\left(\mathrm{NaNO}_2+\mathrm{HCl}\right)$ at $T \leqslant 10^{\circ} \mathrm{C}$ step 2: boil/heat in water
(ii)
Question
Separate samples of 0.02mol of calcium carbonate and 0.02mol of barium carbonate are heated until completely decomposed to the metal oxide and carbon dioxide.
(a) State which of these two Group 2 carbonates requires the higher temperature before it begins to decompose. Explain your answer.
(b) After decomposition is complete, the 0.02mol sample of calcium oxide is taken and added to 2.00dm³ of water. A solution is formed with no solid present. Dilute sulfuric acid is then added dropwise until a precipitate is seen.
The same procedure is repeated with the 0.02mol sample of barium oxide, using the same concentration solution of dilute sulfuric acid.
Identify the sample to which most sulfuric acid must be added to cause a precipitate to appear.
Explain your answer. You should refer to the solubilities of the precipitates and relevant energy terms in your answer.
(c) (i) Calculate the mass, in g, of CO2 produced by the decomposition of 0.020 moles of calcium carbonate.
(ii) Calculate the minimum mass, in g, of propane that would, on complete combustion, produce the same mass of CO2 calculated in (c)(i).
Give your answer to three significant figures.
Answer/Explanation
Answer (a) • barium carbonate / Ba / BaCO3
• larger ionic radius OR smaller charge density of cation / M2+
• anion / CO32– / carbonate ion is less distorted / less polarised OR C-O / C=O less weakened
(b) • calcium oxide / calcium hydroxide
• CaSO4 / calcium sulfate is more soluble OR BaSO4 is less soluble
• ΔHlatt and ΔHhyd are less exothermic / more endothermic (for BaSO4) • ΔHhyd is dominant factor / ΔHhyd change is greater OR ΔHlatt changes less
(c)(i) mass of CO2 = 0.02 × 44 = 0.88 g
(c)(ii) (writes correct equation, deduces 3 CO2 per mole)
moles of propane = 0.02 / 3 OR 0.00667 OR 1 / 150
mass of propane = 0.02 / 3 × 44 = 0.293 g ecf M1 × 44 3sf needed