Question
The diagram shows part of the structure of polymer X.
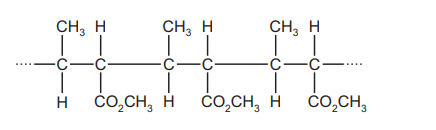
Which reagents react with polymer X?
1 aqueous sulfuric acid
2 aqueous sodium hydroxide
3 sodium
▶️Answer/Explanation
Ans:B
Question
Which statement is correct when referring to the complete combustion of PVC?
A A gas is made which contributes to global warming.
B Carbon dioxide and water are the only products.
C If water is used to clean the exhaust gases, the water becomes alkaline.
D There is no need to treat the exhaust gases as the products are non-hazardous.
▶️Answer/Explanation
Ans:A
Question
A mixture of the three isomers of \(C_{2}H_{2}C \imath_{2}\) is polymerised.
Which sequences will be seen within the polymer chains?
▶️Answer/Explanation
Ans:A
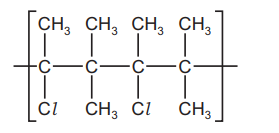
Question:
A section of a polymer chain is shown.
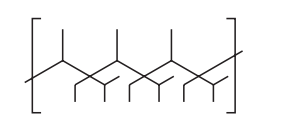
What is the correct monomer?
▶️Answer/Explanation
Ans:A
Question
Which statement about poly(chloroethene) is correct?
A The polymer can be cracked to produce chlorinated alkenes.
B The polymer has harmless combustion products.
C The polymer is readily biodegradable when buried.
D The repeat unit of the polymer has an $M_{\mathrm{r}}$ of 97 .
▶️Answer/Explanation
Ans:A
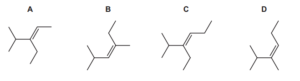
Question
A section showing two repeat units of an addition polymer is shown.
What is the identity of the monomer that produced this polymer?
A 2-chloro-3-methylbutane
B 2-chloro-3-methylbut-2-ene
C 2-chloropent-2-ene
D 2,4-dichloro-3,3,4,5-tetramethylhexane
▶️Answer/Explanation
Ans:B
Question
A molecule of a polymer contains the sequence shown.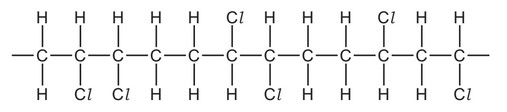
Which monomer could produce this polymer by addition polymerisation?
A CHCl=CHCl
B \(CH_2=CHCl\)
C \(CH_3CCl=CHCl\)
D \(CH_3CCl=CH_2\)
Answer/Explanation
Ans: B
Question
The diagram shows a section of an addition polymer formed from two different monomers.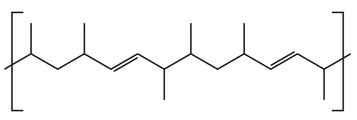
One of the monomers is propene.
What is the other monomer?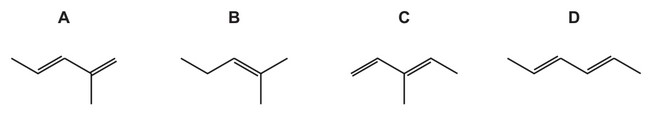
Answer/Explanation
Ans: D
Question
Polymer Z contains the length of polymer chain shown below.
This short length of chain is found many times within the chains of polymer Z, although it is not
the repeat unit.
![]()
What could be the name of polymer Z?
1 poly(2-chloropropene)
2 poly(chloroethene)
3 PVC
Answer/Explanation
Answer C
Question
Poly(ethene) and PVC are examples of addition polymers.
Which statements are correct?
1 On combustion, PVC can produce carbon monoxide, carbon dioxide and hydrogen chloride.
2 When poly(ethene) is buried in a landfill site, it will not significantly biodegrade.
3 The empirical formula of an addition polymer is the same as that of the monomer.
The responses A to D should be selected on the basis of
Answer/Explanation
Answer:
A
Question
A section of an addition polymer chain is shown.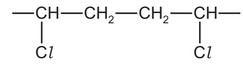
Which monomer could be used to make this polymer?
A \(CH_2CHCH_2Cl\)
B \(CH_2CHCl\)
C \(CH_3CHCHCl\)
D \(CHCl CHCH_2CH_2Cl\)
Answer/Explanation
Ans: B
Question
The diagram shows a short length of an addition polymer chain.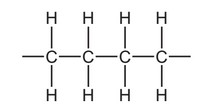
The polymer has a relative molecular mass of approximately 10 000.
Approximately how many monomer units are joined together in each polymer molecule?
A 180 B 360 C 625 D 710
Answer/Explanation
Ans: B
Question
PVC is difficult to dispose of. Two possible methods are burying it in landfill sites and disposal by combustion.
Which row of the table is correct?
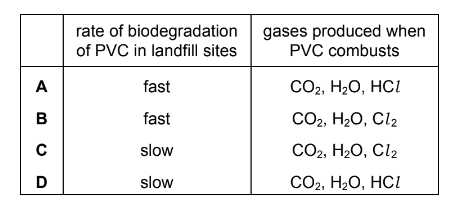
Answer/Explanation
Answer: D
Question
The diagram shows the structure of an addition polymer, X.
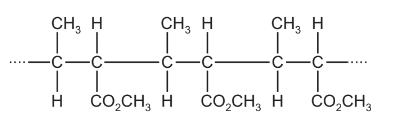
Which reagents react with polymer X?
1 aqueous sulfuric acid
2 aqueous sodium hydroxide
3 sodium
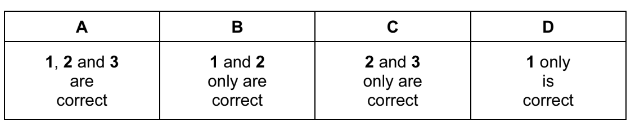
Answer/Explanation
Answer: B
Question
Some vegetable oils contain ‘trans fats’ that are associated with undesirable increases in the amount of cholesterol in the blood.
In the diagrams below, R1 and R2 are different hydrocarbon chains.
Which diagram correctly illustrates an optically active ‘trans fat’?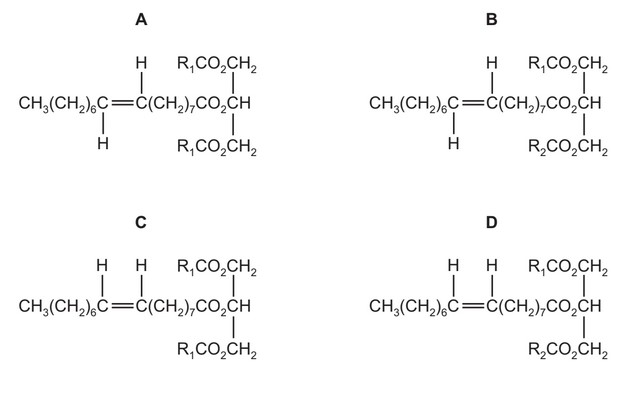
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: B
Question
Which types of bond breakage and bond formation occur in the addition polymerisation of alkenes?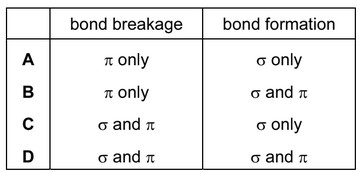
Answer/Explanation
Ans: A
Question
Which statement does not correctly describe the polymer PVC?
- Combustion of PVC waste produces a highly acidic gas.
- PVC molecules are saturated.
- The empirical formula of PVC is the same as the empirical formula of its monomer.
- The repeat unit of PVC is –(CHClCHCl)–.
Answer/Explanation
Ans:
D