Question
Compounds J and K are found in plant oils.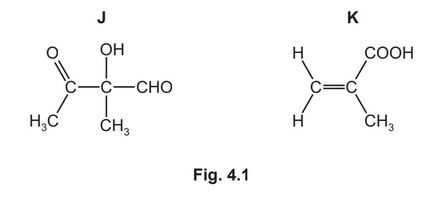
(a) (i) Complete Table 4.1 to state what you would observe when J reacts with the reagents listed.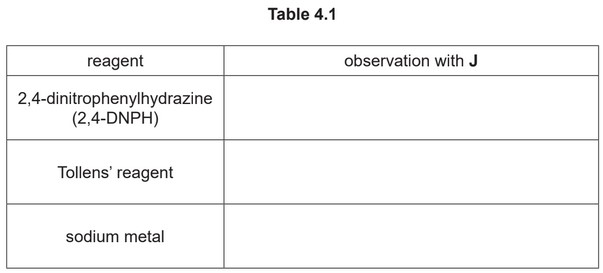
(ii) J has two optical isomers.Draw the three-dimensional structures of the two optical isomers of J.
(b) K is used to make the addition polymer Perspex®. A synthesis of Perspex® is shown in Fig. 4.2.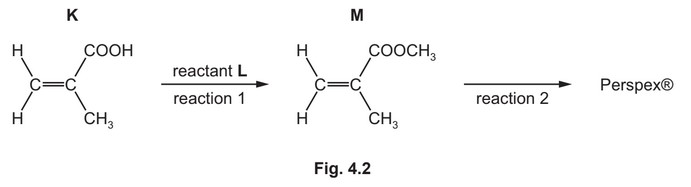
(i) Identify L. State the conditions required for reaction 1.
L =
conditions =
(ii) Draw one repeat unit of the addition polymer Perspex®.
(iii) Use information from Table 4.2 to suggest how the infrared spectra of M and Perspex® would differ. Explain your answer.
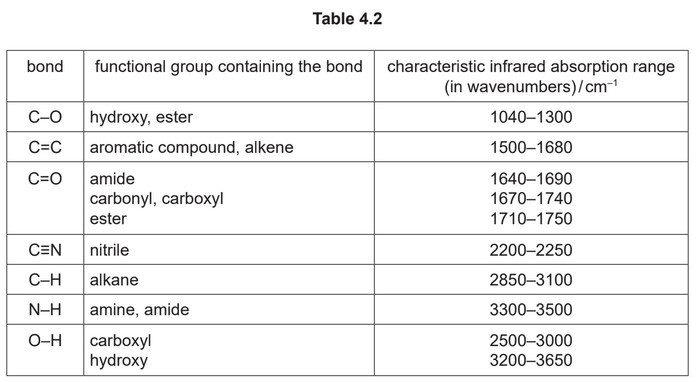
(iv) K can be made from propanone in the three-step synthesis shown in Fig. 4.3.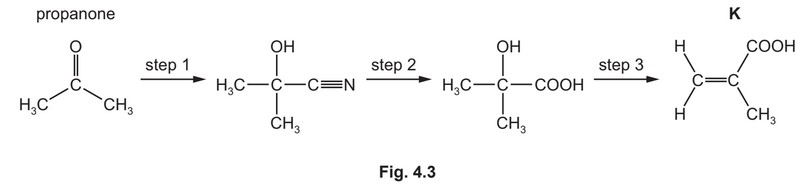
Complete Table 4.3 to identify the reagent(s) used and the type of reaction in each step.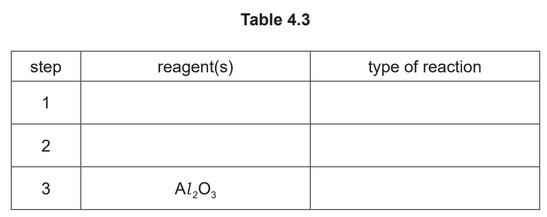
Answer/Explanation
Answer:
(a) (i) red / orange / yellow precipitate / ppt / solid
silver mirror / silver / grey solid / precipitate / ppt
effervescence / bubbling / fizzing
(ii) 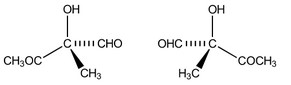
(b) (i) L = \(CH_3OH\) / methanol
conditions = acid(ic) / H+ / \(H_2SO_4\) AND (heat under) reflux
(ii) 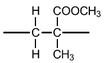
carbon backbone with ‘dangling’ bonds
rest of structure correct
(iii) Perspex® would not have absorption 1500 –1680 cm–1 AND Perspex® does not have C=C
(iv) step 1 KCN / HCN OR NaCN / \(H_2SO_4\) addition
step 2 \(H^+ / H_2SO_4(aq)\) hydrolysis / substitution
step 3 elimination / dehydration