Question:
When reactant X is treated with a suitable reagent, products Y and Z are formed. Infrared spectra of X, Y and Z are shown.
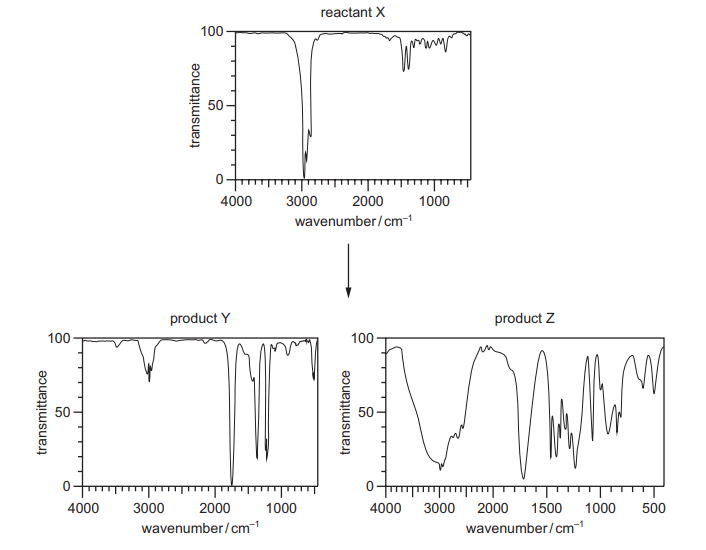
Which row could be correct?
▶️Answer/Explanation
Ans:B
Question
The structure of compound R is shown.
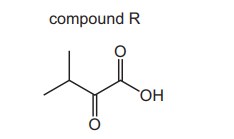
Which statements about compound $\mathrm{R}$ are correct?
1 It has an $M_{\mathrm{r}}$ of 116 .
2 It contains two groups that show strong absorptions between 1640 and $1740 \mathrm{~cm}^{-1}$ in its infrared spectrum.
3 Its only infrared absorption between 2500 and $3000 \mathrm{~cm}^{-1}$ is sharp and strong.
▶️Answer/Explanation
Ans:B
Question
Compound X has the infra-red spectrum shown.
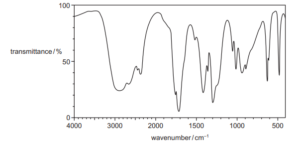
What could be the identity of compound X?
A ethanoic acid
B ethanol
C ethylethanoate
D propanone
▶️Answer/Explanation
Ans:A
Question:
The infrared spectrum shown was obtained from a compound J.
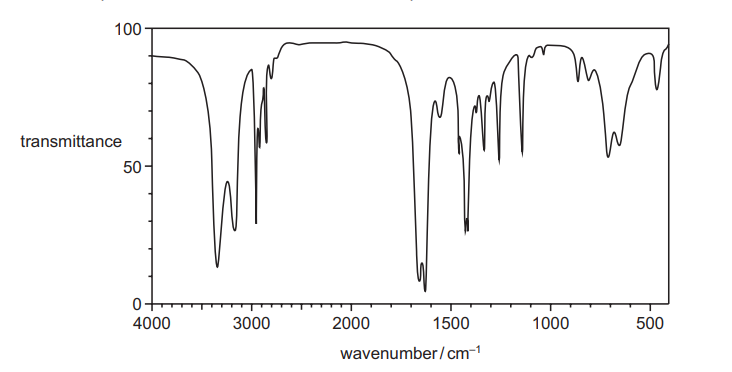
Which statement about J is correct?
A Both C=O and C≡N are present.
B Neither C=O nor C≡N are present.
C C=O is present but not C≡N.
D C≡N is present but not C=O.
▶️Answer/Explanation
Ans:C
Question
Which diagram shows the infrared spectrum of a compound that contains both a C=O and an O–H group?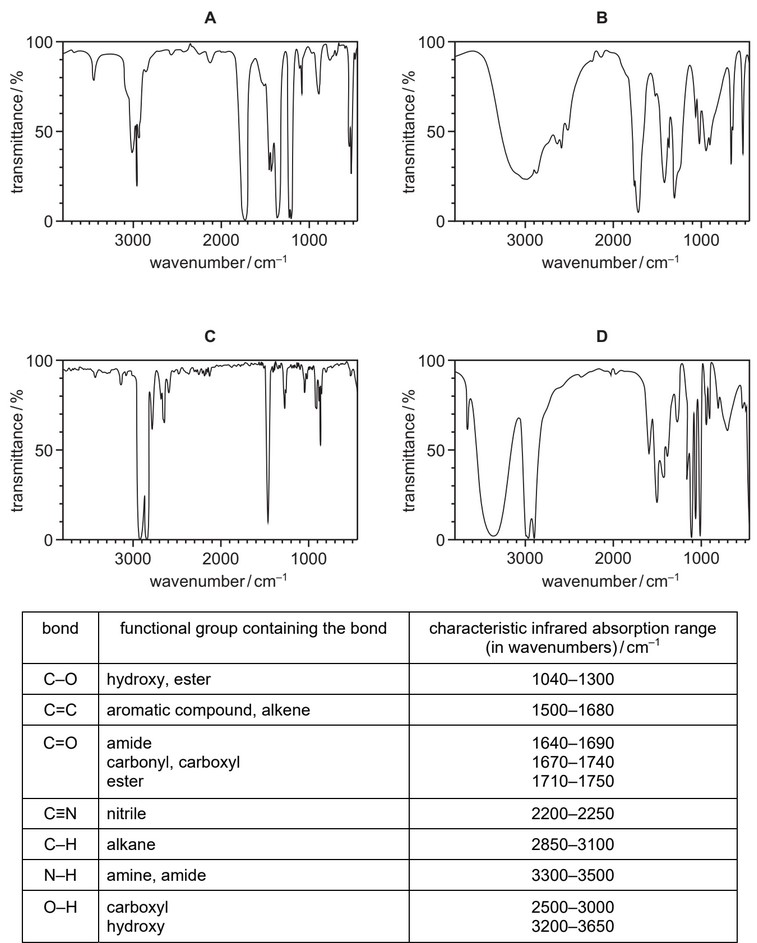
Answer/Explanation
Ans: B
Question
Compound X has the empirical formula C2H4O. Its infra-red spectrum is shown.
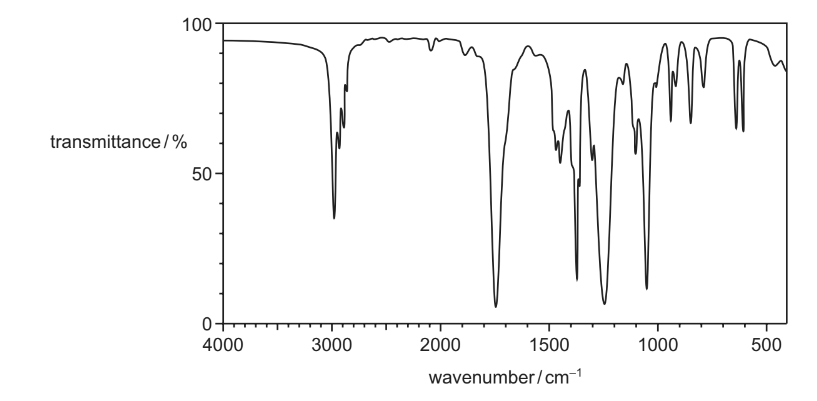
What could be the skeletal formula of compound X?
Answer/Explanation
Answer: A
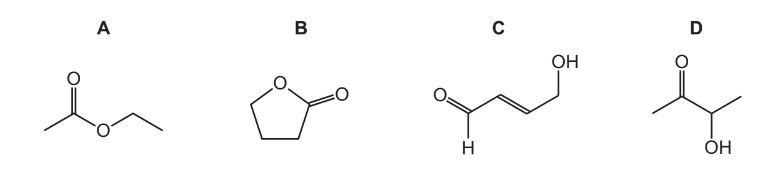
Question
The infra-red spectra of three organic compounds are shown.
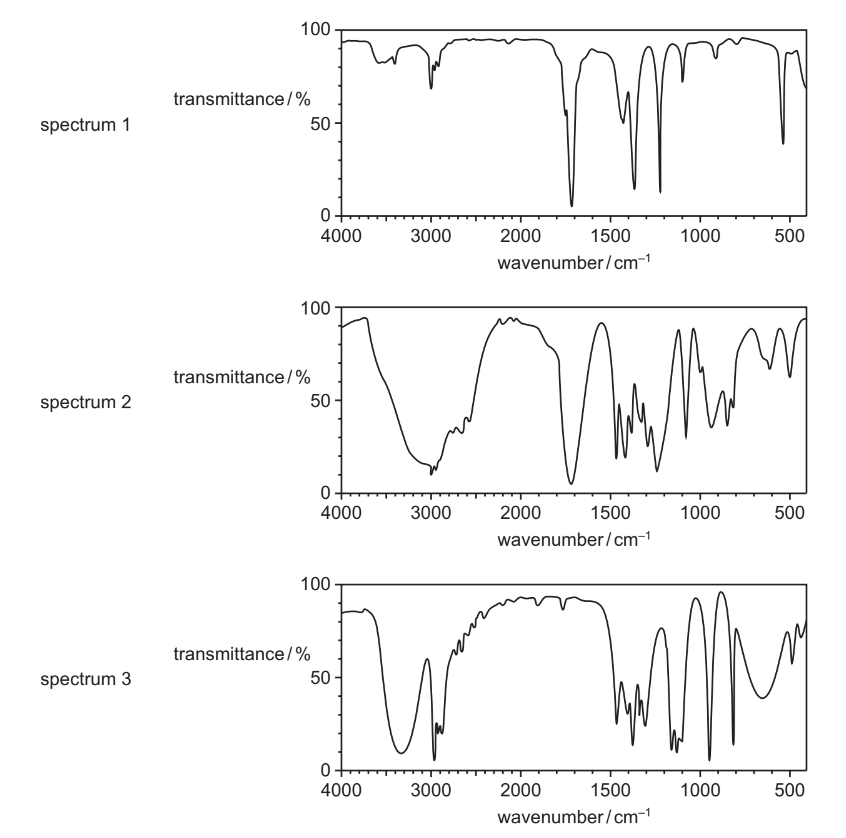
What could the three compounds be?
Answer/Explanation
Question
An infra-red spectrum shows a broad peak at 3000 cm–1 and a strong peak at 1710 cm–1.
Which substance could have produced this spectrum?
A methyl propanoate
B propan-2-ol
C propanoic acid
D propanone
Answer/Explanation
Answer:
C
Question
The infra-red spectrum of compound P is shown.
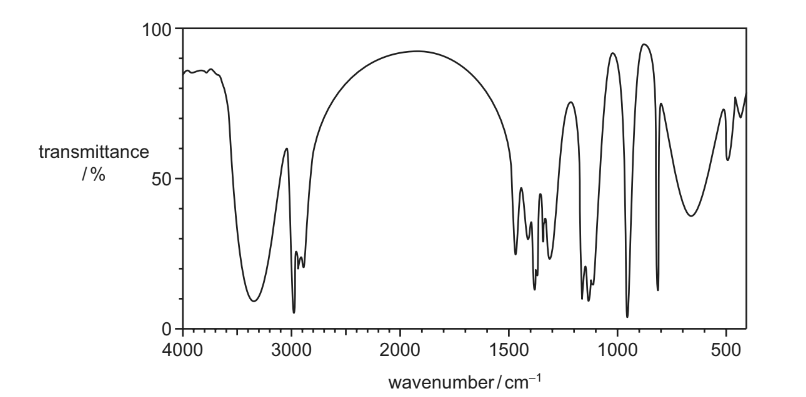
What could be compound P?
A methyl ethanoate
B propanal
C propanoic acid
D propan-2-ol
Answer/Explanation
Answer D
Question
The infra-red spectrum of a substance with empirical formula C2H4O is shown.
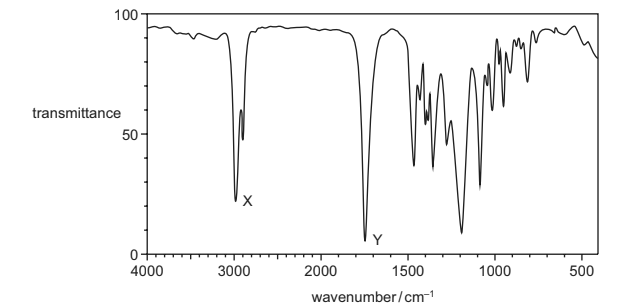
Which bonds are responsible for peak X and peak Y?
Answer/Explanation
Answer B
Question
J is a branched-chain alcohol, C5H12O. J is heated under reflux with an excess of Cr2O72– /H+ until no further reaction occurs. An organic compound K is formed in good yield.
The infra-red spectrum of K is shown.
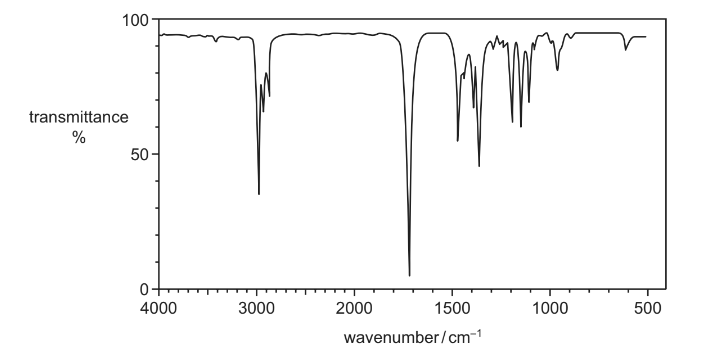
What are the structures of the branched-chain alcohol J and compound K?
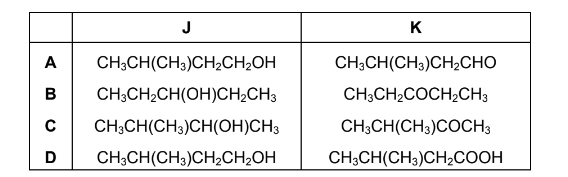
Answer/Explanation
Answer C
Question
Compound X contains three carbon atoms. Part of a simplified infra-red spectrum of compound X is shown.
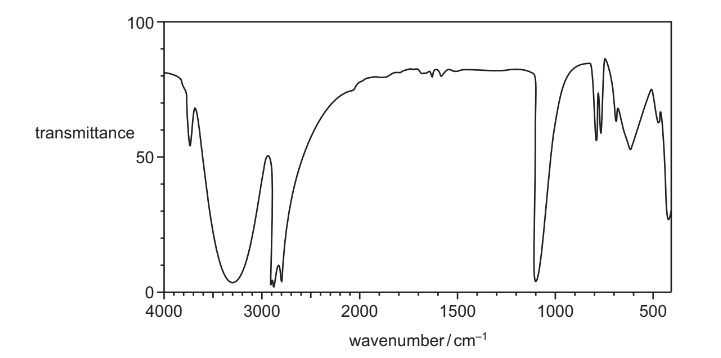
Which compound could be X?
A CH3CH2CHO
B CH3CH2CO2H
C CH3CH2CH2OH
D CH3CO2CH3
Answer/Explanation
Answer C
Question
How many structural isomers with the molecular formula C4H10O give infra-red absorptions both at approximately 1200 cm–1 and at approximately 3400 cm–1?
A 2 B 4 C 6 D 7
Answer/Explanation
Answer:
B
Question
Beams of charged particles are deflected by an electric field. In identical conditions the angle of deflection of a particle is proportional to its charge / mass ratio.
In an experiment, protons are deflected by an angle of +15°. In another experiment under identical conditions, particle Y is deflected by an angle of –5°.
What could be the composition of particle Y?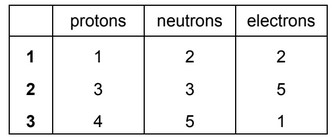
The responses A to D should be selected on the basis of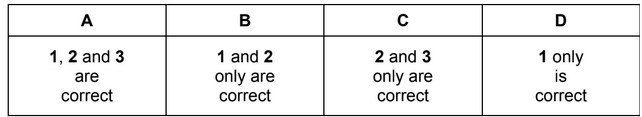
Answer/Explanation
Ans: B