Question
Compound F contains the elements carbon, hydrogen and oxygen only. All carbon-carbon bonds in F are single bonds. The structure of F was analysed by mass spectrometry and infra-red and NMR spectroscopy.
(a) The mass spectrum shows that the m/e value for the M peak is 90. The ratio of the heights of the M and M+1 peaks is 22.1:0.7.
(i) Use the ratio of the heights of the M and M+1 peaks to calculate the number of carbon atoms in a molecule of F.
(ii) Suggest the molecular formula of F.
molecular formula = C H O
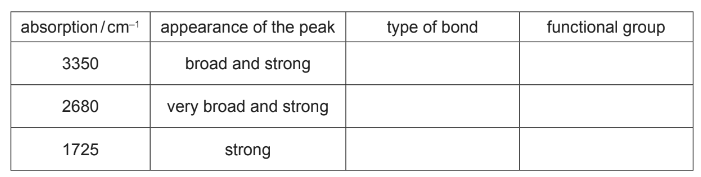
(b) The infra-red spectrum of F was obtained.
Use the Data Booklet and your knowledge of infra-red spectroscopy to identify the type of bond and the functional group responsible for these three absorptions.
(c) F was dissolved in deuterated trichloromethane, CDCl3, and the proton NMR spectrum of this solution obtained.
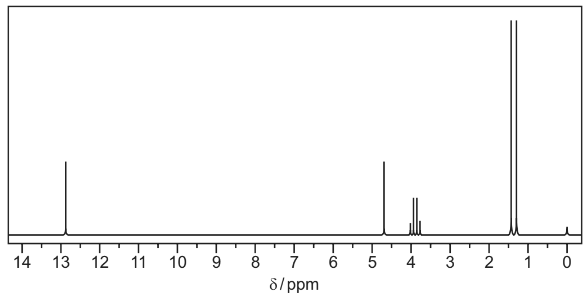
(i) Use the Data Booklet and your answer to (a)(ii) to complete Table 1 for the proton NMR
spectrum of F.
The actual chemical shifts for the four absorptions in F have been added for you.
(ii) Describe and explain the splitting pattern for the absorption at δ = 1.4.
(iii) F was dissolved in D2O and the proton NMR spectrum of this new solution obtained.
Two of the absorptions in Table 1 were not present in this spectrum.
Which absorptions were not present?
(iv) Suggest the structure of F.
(d) Molecules of cycloheptadiene, C7H10, consist of a seven-membered ring with two
carbon-carbon double bonds.
(i) Complete the skeletal formulae of two isomers of cycloheptadiene.
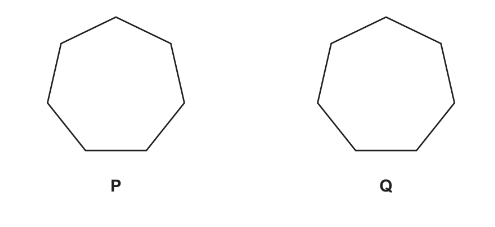
The isomers P and Q were analysed using carbon-13 NMR spectroscopy.
(ii) Predict the number of peaks that will be seen in the carbon-13 NMR spectra of P and Q.

Answer/Explanation
Answer: (a)(i) 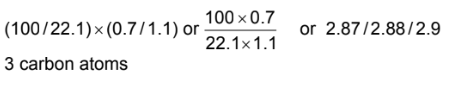
(a)(ii) C3H6O3
(b) 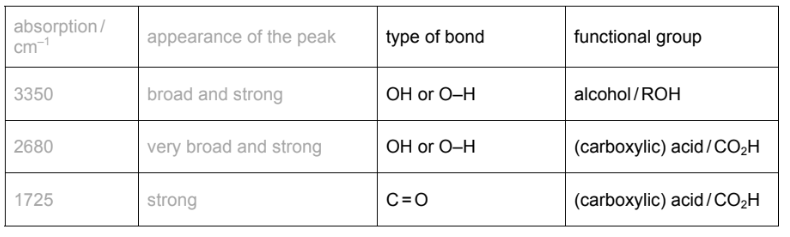
(c)(i) 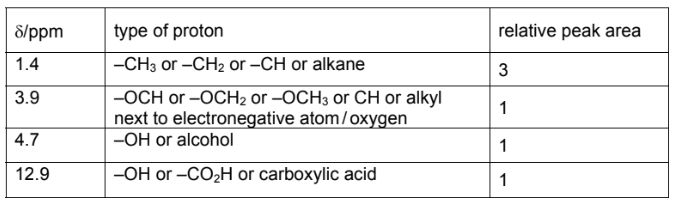
(c)(ii) doublet and 1 / one H/ proton on neighbouring OR adjacent carbon
(c)(iii) 4.7 and 12.9 OR –OH and –CO2H
(c)(iv) 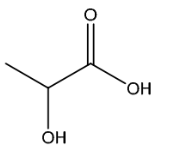
(d)(i) 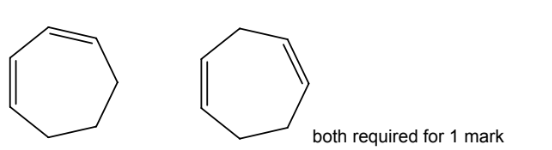
(d)(ii) 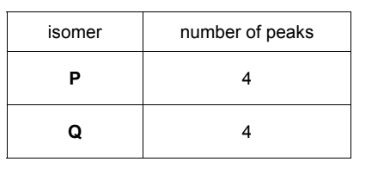
Question
(a) Many common drugs are taken orally, but some medications, such as those based on protein molecules, are injected to prevent them being broken down in the digestive system.
(i) Name a functional group present in drug molecules that might be broken down by acid in the stomach.
(ii) State the type of reaction that would cause such a breakdown.
(iii) Which one of the following compounds would not be suitable to be taken orally?
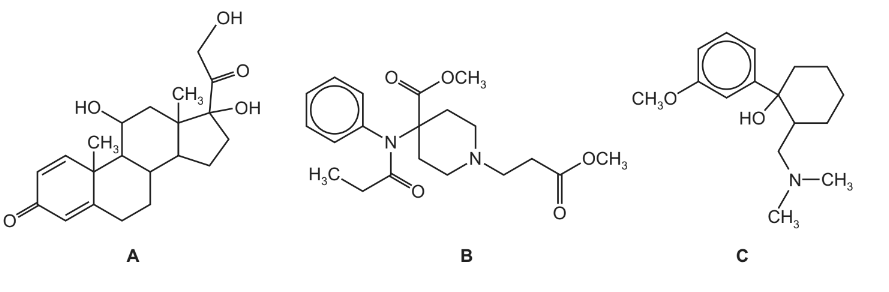
(iv) On the structure of your chosen compound in (iii), circle all the functional groups that might be broken down by acid.
(b) One way of protecting drug molecules that are taken orally is to enclose them in liposomes. These are artificially created spheres made from phospholipids which have an ionic phosphate ‘head’ and two hydrocarbon ‘tails’.
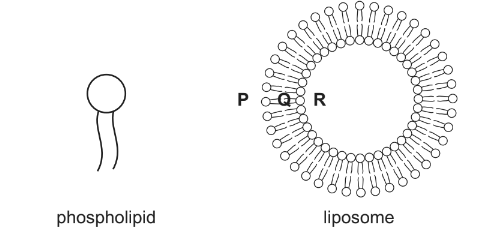
(i) State and explain in which location, P, Q or R, a hydrophobic drug could be carried.
(ii) By considering the nature of the functional groups in A, B and C, explain why these drugs can be carried at position R in the liposome.
(c) Another method of protecting drug molecules is to ‘trap’ them inside gold nano-cages. When they reach the site where they are needed, such as a tumour, the drug is released by exposing the site to infra-red radiation.
(i) Suggest the size of the nano-cages in metres.
(ii) Suggest why infra-red, rather than higher frequency radiation is used.
▶️Answer/Explanation
Ans:
(a) (i) Amide or ester or peptide[1]
(ii) Hydrolysis[1]
(iii) Drug $\mathbf{B}$[1]
(iv) two ester and one amide groups circled
(b) (i) At point $\mathbf{Q}$ because the hydrocarbon tails region is hydrophobic/non-polar/ form van der Waals only
[1] or can dissolve in the fat-soluble area
(ii) They all contain polar or hydrogen-bonding (groups)[1]
(c) (i) range $1 \times 10^{-9}$ to $1 \times 10^{-7} \mathrm{~m}$
(ii) (higher frequency radiation could) cause tissue/cell damage or mutation or harmful to cells[1] [Total: 9]