Question
A scientist chooses either infrared spectroscopy or mass spectrometry to find a particular piece of information.
In which row has the best choice been made?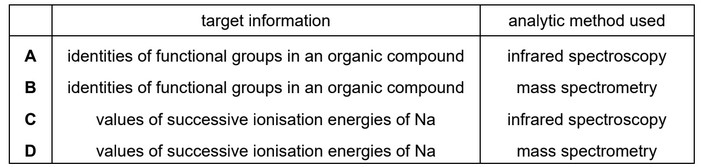
Answer/Explanation
Ans: A
Question
The mass spectrum of a sample of neon is shown. The relative abundance of each peak is written in brackets above it.
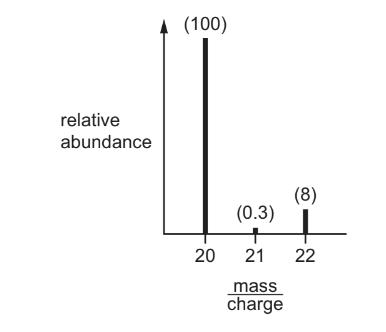
What is the relative atomic mass, Ar, of this sample of neon?
A. 20.15 B. 20.20 C. 21.00 D. 21.82
Answer/Explanation
Answer A
Question
A sample of element X is analysed using mass spectrometry. The mass spectrum obtained is shown.
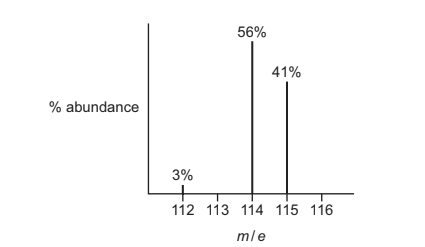
What is the relative atomic mass of this sample of element X?
A 113.7 B 114.0 C 114.2 D 114.4
Answer/Explanation
Answer D
Question
A sample of boron contains aluminium as the only impurity. A mass spectrum of the mixture shows three lines corresponding to three ions, X+ , Y+ and Z+.
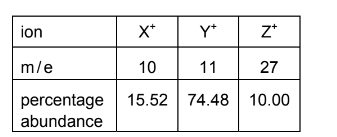
Which statements are correct?
1 There are more electrons in Z+ than in X+. 2 The Ar of boron in the sample is 10.83 to four significant figures.
3 There are more protons in Y+ than in X+.
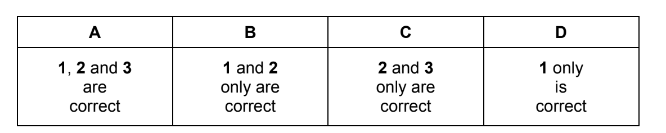
Answer/Explanation
Answer: B