Question
(a) Identify the substances liberated at the anode and at the cathode during the electrolysis of aqueous sodium sulfate, $\mathrm{Na}_2 \mathrm{SO}_4(\mathrm{aq})$.
anode……………………………….
cathode………………………………..[1]
(b) When molten sodium chloride is electrolysed, chlorine is liberated at the anode and sodium is liberated at the cathode.
A sample of molten sodium chloride is electrolysed for 1.50 hours using a current of $4.50 \mathrm{~A}$.
Calculate the volume of chlorine and the mass of sodium that are liberated under room conditions.
volume of chlorine $=$ $\mathrm{dm}^3$
mass of sodium $=$g [4]
(c) The equation representing the standard electrode potential, $E^{\ominus}$, for the reduction of $\mathrm{MnO}_4^{-}(\mathrm{aq})$ to $\mathrm{Mn}^{2+}(\mathrm{aq})$ in acid solution is given.
$
\mathrm{MnO}_4^{-}(\mathrm{aq})+8 \mathrm{H}^{+}(\mathrm{aq})+5 \mathrm{e}^{-} \rightleftharpoons \mathrm{Mn}^{2+}(\mathrm{aq})+4 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \quad E^{\ominus}=+1.52 \mathrm{~V}
$
(i) Draw a diagram of the apparatus that would be used to measure the $E^{\ominus}$ value of this half-cell. Your diagram should be fully labelled to identify all apparatus, substances and conditions.[4]
(ii) Use the Data Booklet to identify a substance that could be used to oxidise $\mathrm{Mn}^{2+}$ ions to $\mathrm{MnO}_4^{-}$ions under standard conditions.
Write an equation for the reaction.[2][Total: 11]
▶️Answer/Explanation
Ans:
(a)
$
\text { (anode }=\text { ) oxygen } / \mathrm{O}_2 \text { AND (cathode }=\text { ) hydrogen } / \mathrm{H}_2
$
BOTH [1]
(b.)
$M 1: Q=1.5 \times 60 \times 60 \times 4.5=24300$ (C) [1]
M2: no. of $\mathrm{F} /$ moles of $e^{-}=24300 / 96500=0.25(1813)[1]$ ecf
M3: volume of $\mathrm{Cl}_2=24 \times 0.252 / 2=3.02 \mathrm{dm}^3[1]$ ecf min $2 \mathrm{sf}$
M4: mass of $\mathrm{Na}=0.252 \times 23=5.79(5.7917) \mathrm{g} \mathrm{Na}[1]$ ecf min $2 \mathrm{sf}$
c)(i)
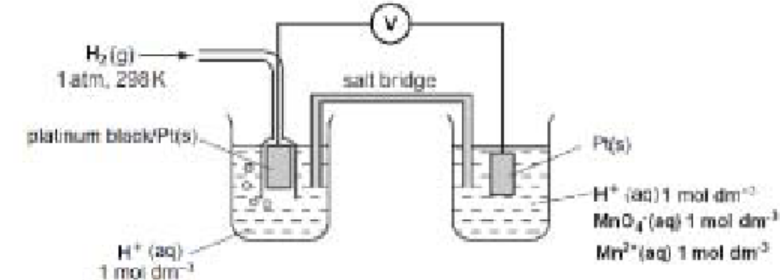
- $\mathrm{MnO}_{4^{-}}, \mathrm{H}^{+}, \mathrm{Mn}^{2+}$ in same beaker AND H${ }^{+}$in other beaker
- both electrodes Pt(s) (ALLOW graphite)
- one solute clearly identified as 1M / 1 mol dm–3
- 298 K OR 1 atm
- voltmeter / potentiometer labelled (or circled V)
- salt bridge labelled (must touch the solution)
- $\quad$ a good delivery system for $\mathrm{H}_2(\mathrm{~g})$
- $\mathrm{H}_2(\mathrm{~g})$
mark as two correct points $=1$ mark [4]
c)(ii) $\begin{aligned} & \mathrm{F}_2 \text { OR } \mathrm{S}_2 \mathrm{O}_8{ }^{2-} \text { OR } \mathrm{H}_2 \mathrm{O}_2 \text { OR HOCl OR } \mathrm{Co}^{3+} \text { OR } \mathrm{Pb}^{4+}[1] \\ & 2 \mathrm{Mn}^{2+}+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{~F}_2 \rightarrow 2 \mathrm{MnO}_4^{-}+16 \mathrm{H}^{+}+10 \mathrm{~F}^{-}[1] \\ & \text { OR } 2 \mathrm{Mn}^{2+}+5 \mathrm{~S}_2 \mathrm{O}_8{ }^{2-}+8 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MnO}_4^{-}+16 \mathrm{H}^{+}+10 \mathrm{SO}_4{ }^{2-} \\ & \text { OR } \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O}+5 \mathrm{Co}^{3+} \rightarrow \mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{Co}^{2+} \\ & \text { OR } 2 \mathrm{Mn}^{2+}+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{~Pb}^{4+} \rightarrow 2 \mathrm{MnO}_4^{-}+16 \mathrm{H}^{+}+5 \mathrm{~Pb}^{2+} \\ & \text { OR } 2 \mathrm{Mn}^{2+}+5 \mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+2 \mathrm{H}_2 \mathrm{O} \\ & \text { OR } 2 \mathrm{Mn}^{2+}+10 \mathrm{HOC} l \rightarrow 2 \mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+5 \mathrm{Cl}_2+2 \mathrm{H}_2 \mathrm{O} \\ & \end{aligned}$
Question
(a) Describe what is meant by a racemic mixture.
(b) Asparagine is an amino acid that contains a chiral carbon atom and displays stereoisomerism.
Separate samples of asparagine are dissolved in \(CDCl_3\) and analysed using carbon-13 and proton (\(^1H\)) NMR spectroscopy.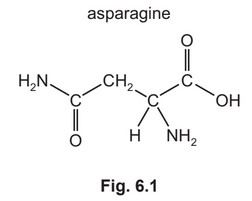
Predict the number of peaks seen in the carbon-13 and proton (\(^1H\)) NMR spectra of asparagine.
(c) The isoelectric point of asparagine, asn, is at pH 5.4.
(i) Describe the meaning of the term isoelectric point.
(ii) Draw the structure of asparagine at pH 1.0.
(d) Asparagine can polymerise to form poly(asparagine).
Draw the structure of poly(asparagine), showing two repeat units. The peptide linkage should be shown displayed.
(e) The isoelectric point of lysine, lys, is at pH 9.8.
A mixture of the dipeptide lys-asn and its two constituent amino acids, asparagine and lysine, is analysed by electrophoresis using a buffer at pH 5.0. The results obtained are shown in
Fig. 6.3.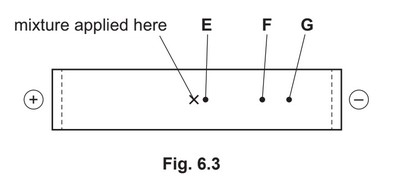
Suggest identities for the species responsible for spots E, F and G. Explain your answers.
(f) Thin-layer and gas-liquid chromatography can be used to analyse mixtures of substances.
Each type of chromatography makes use of a stationary phase and a mobile phase.
(i) Complete Table 6.1 with an example of each of these.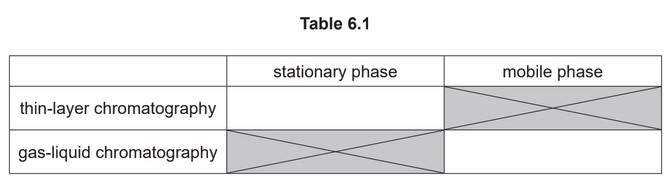
(ii) An unknown amino acid is analysed using thin-layer chromatography. Two chromatographs
of the unknown amino acid and four reference amino acids, P, Q, R and S, are obtained
using two different solvents.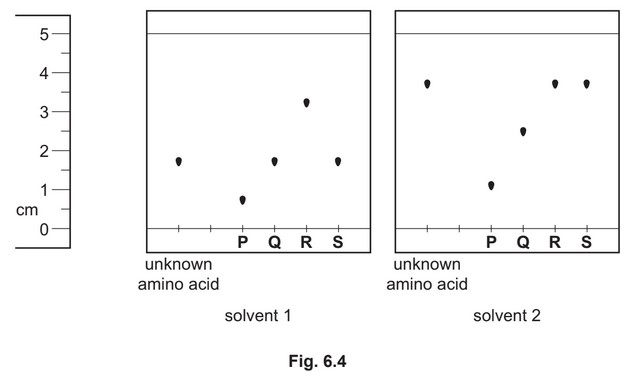
Identify the unknown amino acid. Justify your answer.
(g) A mixture containing three organic compounds is analysed by gas chromatography and mass spectrometry. The gas chromatogram is shown.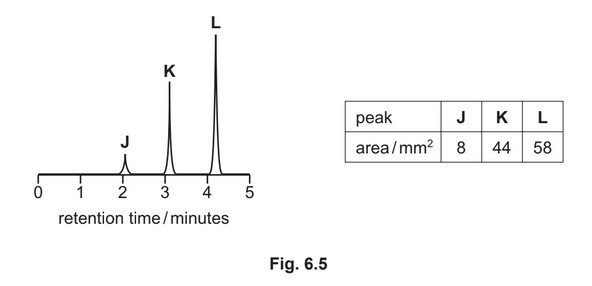
The area underneath each peak is proportional to the mass of the respective compound in the
mixture.
The concentration of K in the mixture is \(5.52 × 10^{–2} gdm^{–3}\).
Calculate the concentration, in moldm–3, of compound L in the mixture.
[\(M_r\): L, 116]
concentration of L = ………………………… \(moldm^{–3}\)
Answer/Explanation
Answer:
(a) a mixture containing equal amounts of each optical isomer
(b) 
(c) (i) the pH at which an amino acid exists as a zwitterion
OR
the pH at which an amino acid has no overall charge
(ii) 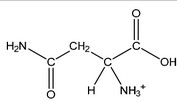
(d) 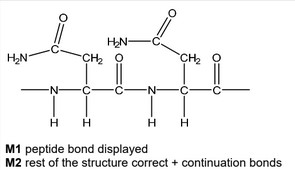
(e) 
M1 table correctly completed
M2 Lys and Lys-Asn are positively charged OR Asn is (nearly) uncharged
M3 LysAsn has the highest \(M_r\)
(f) (i) aluminum oxide / silica (on solid support) AND inert gas / named inert gas e.g. \(N_2\)
(ii) S AND
\(R_f\) is the same as the unknown amino acid in both solvents
(g) mass of L = 58 / 44 * 5.52 \times 10^{–2} = 7.28
\times 10^{–2}\) g
conc. of L = 7.28 \times 10^{–2} / 116 = 6.27 \times 10^{–4} (mol dm^{–3}) min 2sf\)