Question
(a) Define transition element.
(b) Sketch the shape of a \(3d_{z^2}\) orbital.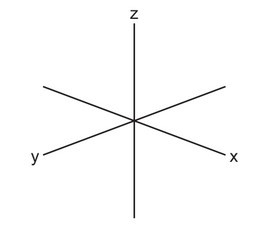
(c) Manganese(IV) oxide, \(MnO_2\), acts as a heterogeneous catalyst in the decomposition of hydrogen peroxide, \(H_2O_2\).
(i) Explain what is meant by a heterogeneous catalyst.
(ii) Describe the mode of action of a heterogeneous catalyst in a reaction.
(d) Manganese(VII) oxide, \(Mn_2O_7\), can be made by treatment of \(KMnO_4\) with concentrated sulfuric acid (reaction 1).
\(Mn_2O_7\) readily decomposes at room temperature to form manganese(IV) oxide and a colourless diatomic gas (reaction 2).
Construct equations for both the reactions described.
reaction 1 …………………………………………………………………………………………………………………..
reaction 2 …………………………………………………………………………………………………………………..
(e) Aqueous manganese(II) ions show similar chemical properties to aqueous copper(II) ions when reacted separately with NaOH(aq) and with concentrated HCl.
(i) Write the ionic equation, and state the type of reaction, for the reaction of \([Mn(H_2O)_6]^{2+}\) with NaOH(aq).
ionic equation ………………………………………………………………………………………………………
type of reaction …………………………………………………………………………………………………….
(ii) Write the ionic equation, and state the type of reaction, for the reaction of \([Mn(H_2O)_6]^{2+}\) with concentrated HCl.
ionic equation ………………………………………………………………………………………………………
type of reaction …………………………………………………………………………………………………….
(iii) Table 2.1 lists relevant electrode potentials for some electrode reactions.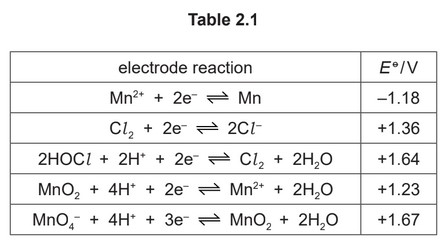
Suggest the formula of the manganese species formed when Mn2+(aq) reacts with Cl2.
State the type of reaction.
formula of manganese species formed ……………………………………………………………………
type of reaction …………………………………………………………………………………………………….
Answer/Explanation
Answer:
(a) forms one or more stable ions / compounds / oxidation states
with incomplete / partially filled (3)d-orbital(s) / d-shell / d-subshell
(b) 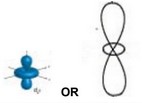
(c) (i) the catalyst and the reactants are in a different state / phase
(ii)
M1 adsorption of reactants to the surface of the catalyst
M2 bonds in the reactants weaken (lowering the activation energy)
M3 reaction occurs and the products are desorbed
(d) M1 reaction 1: \(2KMnO_4 + H_2SO_4 \rightarrow Mn_2O_7 + H_2O + K_2SO_4\)
OR \(2KMnO_4 + 2H_2SO_4 \rightarrow Mn_2O_7 + H_2O + 2KHSO_4\)
M2 reaction 2: \(Mn_2O_7 \rightarrow 2MnO_2 + 1.5O_2\)
(e) (i) M1 \([Mn(H_2O)_6]^{2+} + 2OH^– \rightarrow Mn(OH)_2 + 6H_2O\)
M2 precipitation / acid-base / deprotonation
(ii) M1 \([Mn(H_2O)_6]^{2+} + 4Cl^– \rightarrow [MnCl_4]^{2–} + 6H_2O\)
M2 ligand exchange / substitution / replacement / displacement
(iii) \(MnO_2\) AND redox
Question
Fumaric acid is a naturally occurring dicarboxylic acid.
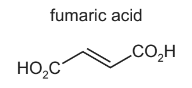
(a) Identify the products of the reaction between fumaric acid and an excess of hot, concentrated, acidified manganate(VII).
(b) Fumaric acid can form addition and condensation polymers.
(i) Draw the repeat unit of the addition polymer poly(fumaric acid).
(ii) Draw the repeat unit of the polyester formed when fumaric acid reacts with ethane-1,2-diol,
(CH2OH)2.
The ester bond should be shown fully displayed.
(iii) Explain why polyesters normally biodegrade more readily than polyalkenes.
(c) Fumaric acid reacts with cold, dilute, acidified manganate(VII) to form compound P.
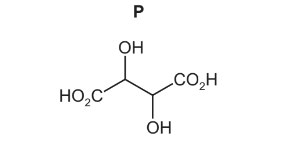
Only three stereoisomers of P exist. One of the stereoisomers is shown.
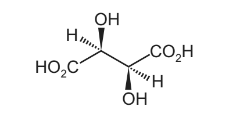
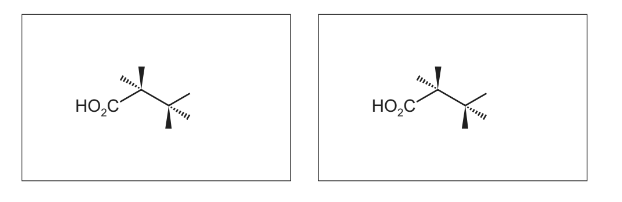
Complete the three-dimensional diagrams in the boxes to show the other two stereoisomers of P.
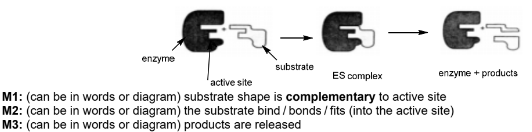
(d) The enzyme fumarase catalyses the reaction of fumarate ions, C4H2O42-, with water to form malate ions, C4H4O52-.
\(C_{4}H_{2}O_{4}^{2-} + H_{2}O \rightleftharpoons C_{4}H_{4}O_{5}^{2-}\)
Describe, with the aid of a suitably labelled diagram, how an enzyme such as fumarase can catalyse a reaction.
Answer/Explanation
Answer: (a) CO2 and H2O / in words
(b)(i) 
(b)(ii) 
(b)(iii) C—C bonds are non-polar / polyalkenes cannot be hydrolysed
OR polyesters / they can be broken down by hydrolysis
(c) 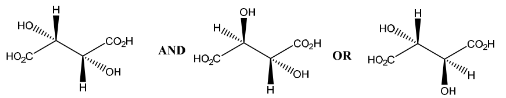
(d)