Question
(a) Group 2 nitrates decompose when heated.
Describe how the thermal stability of Group 2 nitrates changes with increasing proton number.
Explain your answer.
(b) Copper(II) nitrate decomposes in a similar manner to Group 2 nitrates.
Write an equation for the decomposition of \(\mathrm{Cu}\left(\mathrm{NO}_3\right)_2\).
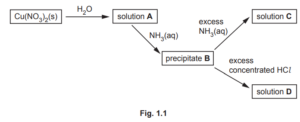
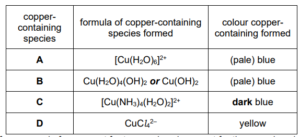
Complete Table 1.1 to show the formula and colour of each of the copper‑containing species present in A, B, C and D.
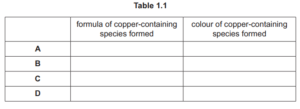
(d) EDTA \({ }^{4-}\) is a polydentate ligand.
(i) Explain what is meant by a polydentate ligand.[2]
(ii) Group 2 metal ions can form complexes similar to those of transition elements.
A solution of EDTA \({ }^{4-}\) is added to water containing \(\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\) to form a new complex, \([\mathrm{CaEDTA}]^{2-}\), as shown.
equilibrium 1
$
\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+\mathrm{EDTA}^{4-} \rightleftharpoons[\mathrm{CaEDTA}]^{2-}+6 \mathrm{H}_2 \mathrm{O}
$
Circle on the structure of EDTA \({ }^{4-}\) in Fig. 1.2 the six atoms that form bonds with the metal ion.
 [1]
[1]
(iii) The calcium ions in \(\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\) and \([\mathrm{CaEDTA}]^{2-}\) have a coordination number of 6 . Explain what is meant by coordination number. [1]
(iv) The complex [CaEDTA \(]^{2-}\) can be used to remove toxic metals from the body.
Table 1.2 shows the numerical values for the stability constants, \(K_{\text {stab }}\), for some metal ions with EDTA \({ }^{4-}\).
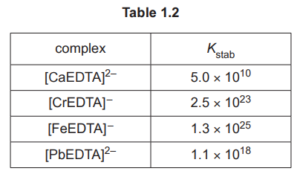
An aqueous solution containing [CaEDTA \(]^{2-}\) is added to a solution containing equal concentrations of \(\mathrm{Cr}^{3+}(\mathrm{aq}), \mathrm{Fe}^{3+}(\mathrm{aq})\) and \(\mathrm{Pb}^{2+}(\mathrm{aq})\). The resulting mixture is left to reach a state of equilibrium.
State the type of reaction when \([\mathrm{CaEDTA}]^{2-}\) reacts with \(\mathrm{Cr}^{3+}(\mathrm{aq}), \mathrm{Fe}^{3+}(\mathrm{aq})\) and \(\mathrm{Pb}^{2+}(\mathrm{aq})\). [1]
(v) Deduce the relative concentrations of \(\left.[\mathrm{CrEDTA}]^{-},{ }_{\mathrm{FeEDTA}}\right]^{-}\)and \([\mathrm{PbEDTA}]^{2-}\) present in the resulting mixture.
Explain your answer.
…………………………………… > …………………………………… > ……………………………………
highest concentration lowest concentration
(e) The number of moles of water of crystallisation in a hydrated ionic salt can be determined by titration using aqueous EDTA \({ }^{4-}\) ions with a suitable indicator.
- \(0.255 \mathrm{~g}\) of hydrated chromium(III) sulfate, \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot n \mathrm{H}_2 \mathrm{O}\), is dissolved in water and made up to \(100 \mathrm{~cm}^3\) in a volumetric flask.
- \(25.0 \mathrm{~cm}^3\) of this solution requires \(26.2 \mathrm{~cm}^3\) of \(0.00800 \mathrm{~mol} \mathrm{dm}^{-3}\) aqueous EDTA \({ }^{4-}\) ions to reach the end-point.
The reaction occurs as shown.
$
\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}+\mathrm{EDTA}^{4-} \rightarrow[\mathrm{CrEDTA}]^{-}+6 \mathrm{H}_2 \mathrm{O}
$
Use the data to calculate the value of \(n\) in the formula of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot n \mathrm{H}_2 \mathrm{O}\).
Show your working.
\(n=\)
(f) A solution of \(\mathrm{Cr}^{3+}(\mathrm{aq})\) and a solution of \(\mathrm{Fe}^{3+}(\mathrm{aq})\) have different colours.
Explain why the two complexes have different colours. [2] [Total: 19]
▶️Answer/Explanation
Ans:
(a) M1 increases (down the group)
M2 radius / size of (cat)ion / \(\mathrm{M}^{2+}\) increases
M3 less polarisation / distortion of anion / nitrate ion / \(\mathrm{NO}_3{ }^{-} \mathrm{OR}\) less weakening of \(\mathrm{N}-\mathrm{O} / \mathrm{N}=\mathrm{O}\) (bond)
(b)\(\mathrm{Cu}\left(\mathrm{NO}_3\right)_2 \rightarrow \mathrm{CuO}+2 \mathrm{NO}_2+1 / 2 \mathrm{O}_2\)
c.
(d)(i) M1 (a species) that donates more than two lone pairs
M2 to form dative / coordinate bonds to a metal atom or ion
(d)(ii) six atoms circled, \(2 \mathrm{~N}\) and \(4 \mathrm{O}\) from different \(\mathrm{CO}_2^{-}\)
(d)(iii) the number of co-ordinate bonds being formed by the metal ion
(d)(iv) ligand exchange
(d)(v) \([\text { FeEDTA }]^{-}>[\text {CrEDTA }]^{-}>[\text {PbEDTA }]^{2-}\)
\(\begin{array}{lll}\text { highest conc } & \text { lowest conc } & \text { AND } K_{\text {stab }} \text { of }[\text { FeEDTA }]^{-} \text {is highest }\end{array}\)
e) M1 moles of \(\mathrm{Cr}^{3+}=2.096 \times 10^{-4}\) in \(25.0 \mathrm{~cm}^3\) )
M2 moles of \(\mathrm{Cr}^{3+}=8.384 \times 10^{-4}\) (in \(100.0 \mathrm{~cm}^3\) )
moles of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \bullet \mathrm{nH}_2 \mathrm{O}=8.384 \times 10^{-4} / 2=4.192 \times 10^{-4}\)
M3 \(M_{\mathrm{r}}\) of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot \mathrm{nH}_2 \mathrm{O}=0.2550 / 4.192 \times 10^{-4}=608.3\) \(\mathrm{n}=(608.3-392.3) / 18=12\)
f) M1 \(\Delta E\) is different M2 different frequency (of light) is absorbed
Question
(a) (i) Describe and explain the trend in the solubility of the Group 2 hydroxides down the group
Group 2 hydroxides decompose on heating to give the corresponding metal oxide and water vapour.
(ii) Suggest which of $\mathrm{Mg}(\mathrm{OH})_2$ and $\mathrm{Sr}(\mathrm{OH})_2$ will decompose at a lower temperature.
Explain your answer.[2] [Total: 6]
▶️Answer/Explanation
Ans:
(a)(i ) M1 solubility increases down the group
M2 $\Delta H_{\text {latt }}$ and $\Delta H_{\text {hyd }}$ both become less exothermic / less negative
M3 $\Delta H_{\text {latt }}$ changes more (than $\Delta H_{\text {hyd }}$ as $\mathrm{OH}^{-}$being smaller than $\mathrm{M}^{2+}$ )
M4 $\Delta H_{\text {sol }}$ becomes more exothermic / more negative
(a)(ii) M1 $\mathrm{Mg}(\mathrm{OH})_2$ AND $\mathrm{Mg}^{2+}$ has a smaller ionic radii/ $\mathrm{Mg}^{2+}$ has a higher charge density $\mathrm{M} 2 \mathrm{OH}^{-}$ion is polarised/distorted more