Question
(a) The electronic configuration of transition element $Q$ is $[\mathrm{Ar}] 3 \mathrm{~d}^2 4 \mathrm{~s}^2$.
Predict the likely oxidation states of element $\mathbf{Q}$ in compounds.[1]
(b) Suggest why transition elements often show variable oxidation states in their compounds, but typical s-block elements such as calcium do not.[1]
(c) Many enzymes contain transition element complexes.
Describe, with the aid of a suitably labelled diagram, how an enzyme catalyses the breakdown of a substrate molecule. [3] [Total: 5]
▶️Answer/Explanation
Ans:
0(a) +4 and any of +1, +2, +3 1
10(b) close similarity of energy of the 4s and 3d sub-shells
(c)
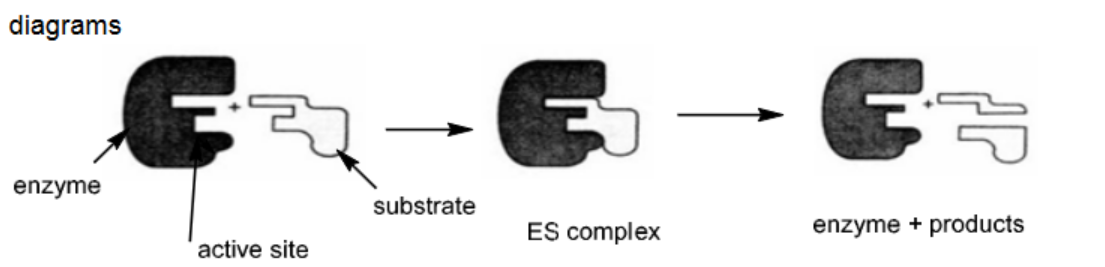
M1 (can be in words or diagram) substrate shape is complementary to active site
M2 (can be in words or diagram) the substrate bind / bonds / fits into the active site
M3 (can be in words or diagram) products are released
M4 (words) lower $E_A /$ bonds weakened (in substrate) Any three points
Question
(a) (i) Complete the following electronic configurations.
- the cobalt atom, Co $1 s^2 2 s^2 2 p^6$
- the cobalt(II) ion, $\mathrm{Co}^{2+} \quad 1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}^6$ [1]
(ii) State the colours you would observe when concentrated $\mathrm{HCl}$ (aq) is added to an aqueous solution of cobalt(II) nitrate, $\mathrm{Co}\left(\mathrm{NO}_3\right)_2$.
Give the formulae and geometry of the complexes formed.
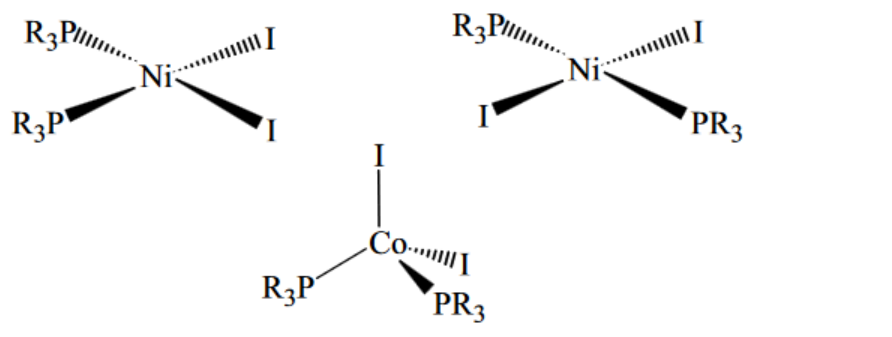
(b) There are two isomers with the formula $\mathrm{Ni}\left(R_3 \mathrm{P}\right)_2 \mathrm{I}_2$, but only one structure with the formula $C o\left(R_3 P\right)_2 I_2$. $\left(R=\right.$ alkyl, $R_3 P$ is a monodentate ligand)
Draw diagrams showing the structure of $\mathrm{Co}\left(\mathrm{R}_3 \mathrm{P}\right)_2 \mathrm{I}_2$ and the two isomers of $\mathrm{Ni}\left(R_3 \mathrm{P}\right)_2 \mathrm{I}_2$.
▶️Answer/Explanation
Ans:
a) (i) $\begin{array}{ll}\text { Co: } & \ldots 3 s^2 3 p^6 3 d^7 4 s^2 \\ \text { Co }^{2+}: & \ldots 3 s^2 3 p^6 3 d^7\end{array}$
(ii) solution starts pink turns blue pink is $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ blue is $\left[\mathrm{CoCl}_4\right]^{2-}$ this complex is tetrahedral
b)