Question
(a) When $1.0 \mathrm{moldm}^{-3} \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(\mathrm{aq})$ is added to a solution containing Ag(aq) ions, a linear complex, $\mathbf{P}$, is formed. $\mathrm{S}_2 \mathrm{O}_3{ }^{2-}$ ions are present in $\mathbf{P}$ as monodentate ligands.
(i) Define the term monodentate ligand.[5]
(ii) Give the formula of $\mathbf{P}$, including its charge.[1]
(b) When $1.0 \mathrm{moldm}^{-3} \mathrm{NaCN}(\mathrm{aq})$ is added to a solution of $\mathbf{P}$, a mixture which includes a second linear complex, $\mathbf{Q}$, is formed. In this mixture the concentration of $\mathbf{Q}$ is much greater than the concentration of $\mathbf{P}$.
(i) Write an equation for the reaction that occurs when $\mathrm{NaCN}(\mathrm{aq})$ is added to a solution of $\mathbf{P}$.[1]
(ii) Suggest a reason why the concentration of $\mathbf{Q}$ is much greater than the concentration of $\mathbf{P}$ in the mixture.
(iii) Name the type of reaction in which $\mathbf{P}$ forms $\mathbf{Q}$. [1]
(c) Platinum forms a complex ion with the formula $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$. In this complex ion the carbon atom of each $\mathrm{CN}^{-}$ligand bonds to the platinum ion. This complex shows stereoisomerism.
(i) There are only two isomers of this complex.
Draw structures of these two isomers in the boxes below.
(ii) Describe the geometry of $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$.[1]
(iii) Name the type of stereoisomerism shown by $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$.[1] [Total: 9]
▶️Answer/Explanation
Ans:
a)(i) (a species) that donates one lone pair [1] to form a dative / coordinate to a central metal atom / metal ion [1]
$6(\mathrm{a})$ (ii) $\quad\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}[1]$
(b)(i) $
\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}+2 \mathrm{CN}^{-} \rightarrow\left[\mathrm{Ag}(\mathrm{CN})_2\right]^{-}+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}[1]
$
OR
$
\left.\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}+2 \mathrm{NaCN} \rightarrow\left[\mathrm{Ag}(\mathrm{CN})_2\right]^{-}+\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3+\mathrm{S}_2 \mathrm{O}_3{ }^{2-}
$
(b)(ii) Q is more stable / has a larger Kstab than P [1]
(b)(iii) ligand exchange / displacement / substitution
(c)(i)
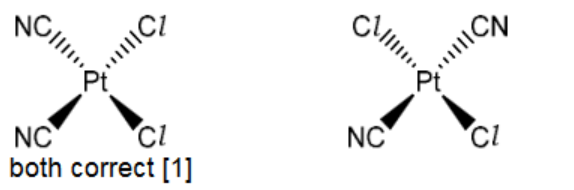
c)(ii) square planar [1] 1
6(c)(iii) cis-trans OR geometric(al) [1]
Question
(a) An aqueous solution of cobalt(II) contains the $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ complex ion.
(i) Define the term complex ion. [1]
(ii) Samples of $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ are reacted separately with aqueous sodium hydroxide and with an excess of aqueous ammonia.
Give the following information about these reactions.
- the reaction of $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ with aqueous sodium hydroxide colour and state of the cobalt-containing species ionic equation type of reaction
- the reaction of $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ with an excess of aqueous ammonia colour and state of the cobalt-containing species ionic equation type of reaction [6],
(b) When concentrated hydrochloric acid is added to a solution containing $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$, a blue solution of $\left[\mathrm{COCl}_4\right]^{2-}$ is formed and the following equilibrium is established.
$
\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+4 \mathrm{Cl}^{-} \rightleftharpoons\left[\mathrm{CoCl}_4\right]^{2-}+6 \mathrm{H}_2 \mathrm{O}
$
Use Le Chatelier’s principle to suggest the expected observations when silver nitrate solution is added dropwise to the blue solution of $\left[\mathrm{CoCl}_4\right]^{2-}$. Explain your answer.
(c) The $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]$ complex shows stereoisomerism.
Complete the three-dimensional diagrams to show the two isomers of $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]$.
Suggest the type of stereoisomerism.

type of stereoisomerism ……………………………………… [2]
(d) Compound $\mathbf{X}, \mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4$, is a tetradentate ligand.
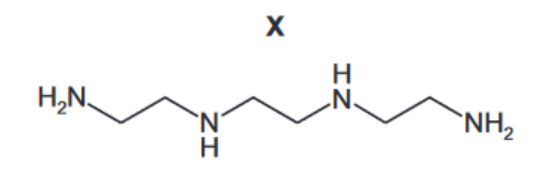
(i) Suggest why one molecule of X can form four dative bonds. [1]
(ii) $\mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4$ reacts with aqueous cobalt(II) ions, $\left[\mathrm{C} \circ\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$, in a $1: 1$ ratio to form a new complex ion.
Construct an equation for this reaction. [1] [Total: 13]
▶️Answer/Explanation
Ans:
(a)(i) (a molecule or ion) formed by a (central) metal atom / ion surrounded by / bonded to (one or more) ligands
a)(ii) M1: blue ppt/solid
M2: $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+2 \mathrm{OH}^{-} \rightarrow \mathrm{Co}(\mathrm{OH})_2+6 \mathrm{H}_2 \mathrm{O}$ OR $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_4(\mathrm{OH})_2\right]+2 \mathrm{H}_2 \mathrm{O}$
M3: precipitation/ acid-base
M4: blue solution
M5: $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+6 \mathrm{NH}_3 \rightarrow\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{2+}+6 \mathrm{H}_2 \mathrm{O}$
M6: ligand exchange/displacement/substitution/replacement
(b) solution turns blue → pink
• a white ppt. of AgCl forms
• equilibrium shifts to the left / [Cl – ] decreases
Two correct responses = 1 mark Three correct responses = 2 marks
(c)
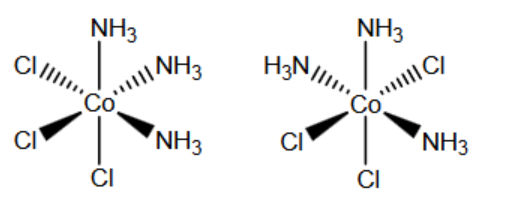
geometric ALLOW cis-trans
Two correct responses = 1 mark Three correct responses = 2 marks
(d)(i) each nitrogen / the four nitrogen’s has a lone pair of electrons (to the metal ion)
Two correct responses = 1 mark
(d)(ii) $\begin{aligned} & {\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+\mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4 \rightarrow\left[\mathrm{Co}\left(\mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4\right)\right]^{2+}+6 \mathrm{H}_2 \mathrm{O}} \\ & \text { OR } \\ & {\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+\mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4 \rightarrow\left[\mathrm{Co}\left(\mathrm{C}_6 \mathrm{H}_{18} \mathrm{~N}_4\right)\left(\mathrm{H}_2 \mathrm{O}\right)_2\right]^{2+}+4 \mathrm{H}_2 \mathrm{O}}\end{aligned}$