Question
(a) Group 2 nitrates decompose when heated.
Describe how the thermal stability of Group 2 nitrates changes with increasing proton number.
Explain your answer.
(b) Copper(II) nitrate decomposes in a similar manner to Group 2 nitrates.
Write an equation for the decomposition of \(\mathrm{Cu}\left(\mathrm{NO}_3\right)_2\).
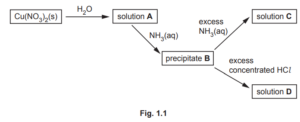
Complete Table 1.1 to show the formula and colour of each of the copper‑containing species present in A, B, C and D.
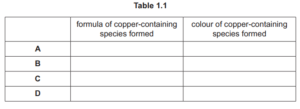
(d) EDTA \({ }^{4-}\) is a polydentate ligand.
(i) Explain what is meant by a polydentate ligand.[2]
(ii) Group 2 metal ions can form complexes similar to those of transition elements.
A solution of EDTA \({ }^{4-}\) is added to water containing \(\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\) to form a new complex, \([\mathrm{CaEDTA}]^{2-}\), as shown.
equilibrium 1
$
\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}+\mathrm{EDTA}^{4-} \rightleftharpoons[\mathrm{CaEDTA}]^{2-}+6 \mathrm{H}_2 \mathrm{O}
$
Circle on the structure of EDTA \({ }^{4-}\) in Fig. 1.2 the six atoms that form bonds with the metal ion.
 [1]
[1]
(iii) The calcium ions in \(\left[\mathrm{Ca}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\) and \([\mathrm{CaEDTA}]^{2-}\) have a coordination number of 6 . Explain what is meant by coordination number. [1]
(iv) The complex [CaEDTA \(]^{2-}\) can be used to remove toxic metals from the body.
Table 1.2 shows the numerical values for the stability constants, \(K_{\text {stab }}\), for some metal ions with EDTA \({ }^{4-}\).
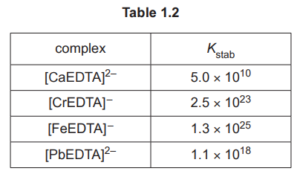
An aqueous solution containing [CaEDTA \(]^{2-}\) is added to a solution containing equal concentrations of \(\mathrm{Cr}^{3+}(\mathrm{aq}), \mathrm{Fe}^{3+}(\mathrm{aq})\) and \(\mathrm{Pb}^{2+}(\mathrm{aq})\). The resulting mixture is left to reach a state of equilibrium.
State the type of reaction when \([\mathrm{CaEDTA}]^{2-}\) reacts with \(\mathrm{Cr}^{3+}(\mathrm{aq}), \mathrm{Fe}^{3+}(\mathrm{aq})\) and \(\mathrm{Pb}^{2+}(\mathrm{aq})\). [1]
(v) Deduce the relative concentrations of \(\left.[\mathrm{CrEDTA}]^{-},{ }_{\mathrm{FeEDTA}}\right]^{-}\)and \([\mathrm{PbEDTA}]^{2-}\) present in the resulting mixture.
Explain your answer.
…………………………………… > …………………………………… > ……………………………………
highest concentration lowest concentration
(e) The number of moles of water of crystallisation in a hydrated ionic salt can be determined by titration using aqueous EDTA \({ }^{4-}\) ions with a suitable indicator.
- \(0.255 \mathrm{~g}\) of hydrated chromium(III) sulfate, \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot n \mathrm{H}_2 \mathrm{O}\), is dissolved in water and made up to \(100 \mathrm{~cm}^3\) in a volumetric flask.
- \(25.0 \mathrm{~cm}^3\) of this solution requires \(26.2 \mathrm{~cm}^3\) of \(0.00800 \mathrm{~mol} \mathrm{dm}^{-3}\) aqueous EDTA \({ }^{4-}\) ions to reach the end-point.
The reaction occurs as shown.
$
\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}+\mathrm{EDTA}^{4-} \rightarrow[\mathrm{CrEDTA}]^{-}+6 \mathrm{H}_2 \mathrm{O}
$
Use the data to calculate the value of \(n\) in the formula of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot n \mathrm{H}_2 \mathrm{O}\).
Show your working.
\(n=\)
(f) A solution of \(\mathrm{Cr}^{3+}(\mathrm{aq})\) and a solution of \(\mathrm{Fe}^{3+}(\mathrm{aq})\) have different colours.
Explain why the two complexes have different colours. [2] [Total: 19]
▶️Answer/Explanation
Ans:
(a) M1 increases (down the group)
M2 radius / size of (cat)ion / \(\mathrm{M}^{2+}\) increases
M3 less polarisation / distortion of anion / nitrate ion / \(\mathrm{NO}_3{ }^{-} \mathrm{OR}\) less weakening of \(\mathrm{N}-\mathrm{O} / \mathrm{N}=\mathrm{O}\) (bond)
(b)\(\mathrm{Cu}\left(\mathrm{NO}_3\right)_2 \rightarrow \mathrm{CuO}+2 \mathrm{NO}_2+1 / 2 \mathrm{O}_2\)
c.
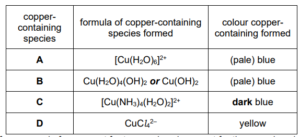
(d)(i) M1 (a species) that donates more than two lone pairs
M2 to form dative / coordinate bonds to a metal atom or ion
(d)(ii) six atoms circled, \(2 \mathrm{~N}\) and \(4 \mathrm{O}\) from different \(\mathrm{CO}_2^{-}\)
(d)(iii) the number of co-ordinate bonds being formed by the metal ion
(d)(iv) ligand exchange
(d)(v) \([\text { FeEDTA }]^{-}>[\text {CrEDTA }]^{-}>[\text {PbEDTA }]^{2-}\)
\(\begin{array}{lll}\text { highest conc } & \text { lowest conc } & \text { AND } K_{\text {stab }} \text { of }[\text { FeEDTA }]^{-} \text {is highest }\end{array}\)
e) M1 moles of \(\mathrm{Cr}^{3+}=2.096 \times 10^{-4}\) in \(25.0 \mathrm{~cm}^3\) )
M2 moles of \(\mathrm{Cr}^{3+}=8.384 \times 10^{-4}\) (in \(100.0 \mathrm{~cm}^3\) )
moles of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \bullet \mathrm{nH}_2 \mathrm{O}=8.384 \times 10^{-4} / 2=4.192 \times 10^{-4}\)
M3 \(M_{\mathrm{r}}\) of \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3 \cdot \mathrm{nH}_2 \mathrm{O}=0.2550 / 4.192 \times 10^{-4}=608.3\) \(\mathrm{n}=(608.3-392.3) / 18=12\)
f) M1 \(\Delta E\) is different M2 different frequency (of light) is absorbed
Question
A solution is made by dissolving $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$ in an excess of aqueous ammonia. This solution contains the copper complex $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2^{2+}}$.
(a) (i) Write an expression for the $K_{\text {stab }}$ of $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}$.
$
K_{\mathrm{stab}}=
$[1]
(ii) State the colour of the solution of $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}$. [1]
The solution of $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}$ is heated gently in a fume cupboard so that $\mathrm{NH}_3$ is released. Some $\mathrm{NH}_3$ remains in solution and some forms $\mathrm{NH}_3$ gas. The colour of the solution changes; a precipitate of $\mathrm{Cu}(\mathrm{OH})_2$ forms and is collected.
A sample of $\mathrm{Cu}(\mathrm{OH})_2$ is added to concentrated hydrochloric acid. A reaction takes place forming a coloured copper complex, Y.
A sample of $\mathrm{Cu}(\mathrm{OH})_2$ is added to dilute sulfuric acid. A reaction takes place forming a coloured copper complex, $\mathbf{Z}$.
$\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}, \mathbf{Y}$ and $\mathbf{Z}$ are different colours.
(b) Suggest an equation for the reaction of $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}$ to form $\mathrm{Cu}(\mathrm{OH})_2$ as the aqueous solution of $\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}$ is heated.[1]
(c) Suggest an equation for the reaction of $\mathrm{Cu}(\mathrm{OH})_2$ with concentrated hydrochloric acid, forming $\mathbf{Y}$. [5]
(d) Complete the table with the colour and geometry of complex $\mathbf{Y}$ and the colour, geometry and formula of complex $\mathbf{Z}$.

e) Explain why complexes Y and Z are coloured and why their colours are different. [5][Total: 12]
▶️Answer/Explanation
Ans:
5(a)(i) $\quad K$ stab $=\left[\left(\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right)^{2+}\right] /\left[\left(\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right)^{2+}\right]\left[\mathrm{NH}_3\right]^4[1]$
5(a)(ii) deep / dark / royal blue [1]
$
\begin{array}{ll}
\text { 5(b) } & {\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Cu}(\mathrm{OH})_2+2 \mathrm{NH}_4+2 \mathrm{NH}_3[1]} \\
& \mathrm{OR}\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Cu}(\mathrm{OH})_2+2 \mathrm{H}^{+}+4 \mathrm{NH}_3 \\
\text { 5(c) } & \left.\mathrm{Cu}(\mathrm{OH})_2+4 \mathrm{HCl} \rightarrow[\mathrm{CuCl})_4\right]^{2-}+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{H}^{+} \\
& \mathrm{OR} \mathrm{Cu}(\mathrm{OH})_2+4 \mathrm{Cl}^{-}+2 \mathrm{H}^{+} \rightarrow\left[\mathrm{CuCl} l_4\right]^{2-}+2 \mathrm{H}_2 \mathrm{O}
\end{array}
$
5(c) $\mathrm{Cu}(\mathrm{OH})_2+4 \mathrm{HCl} \rightarrow\left[\mathrm{CuCl}_4\right]^{2-}+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{H}^{+}$
OR Cu $(\mathrm{OH})_2+4 \mathrm{Cl}^{-}+2 \mathrm{H}^{+} \rightarrow\left[\mathrm{CuCl}_4\right]^{2-}+2 \mathrm{H}_2 \mathrm{O}$
[CuCl4 $]^{2-}$ complex including charge [1] rest of equation fully correct [1]
(d)
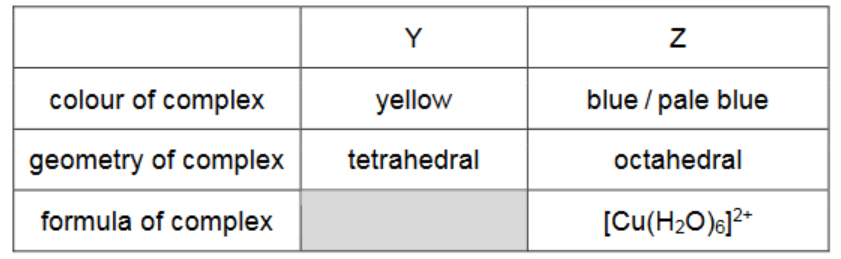
one mark for any three cells [1]
two marks for all five cells [2]
2
5(e) M1: d orbitals splits into two sets of energy levels of different energy [1]
M2: wavelength / frequency / light / photon / hν absorbed [1]
M3: electron(s) promoted / excited [1]
M4: colour seen is complementary (to colour absorbed) [1]
M5: d–d energy gap / ΔE is different for Y and Z
AND so different frequency / wavelength of light absorbed [1]