Question
(a) When $1.0 \mathrm{moldm}^{-3} \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(\mathrm{aq})$ is added to a solution containing Ag(aq) ions, a linear complex, $\mathbf{P}$, is formed. $\mathrm{S}_2 \mathrm{O}_3{ }^{2-}$ ions are present in $\mathbf{P}$ as monodentate ligands.
(i) Define the term monodentate ligand.[5]
(ii) Give the formula of $\mathbf{P}$, including its charge.[1]
(b) When $1.0 \mathrm{moldm}^{-3} \mathrm{NaCN}(\mathrm{aq})$ is added to a solution of $\mathbf{P}$, a mixture which includes a second linear complex, $\mathbf{Q}$, is formed. In this mixture the concentration of $\mathbf{Q}$ is much greater than the concentration of $\mathbf{P}$.
(i) Write an equation for the reaction that occurs when $\mathrm{NaCN}(\mathrm{aq})$ is added to a solution of $\mathbf{P}$.[1]
(ii) Suggest a reason why the concentration of $\mathbf{Q}$ is much greater than the concentration of $\mathbf{P}$ in the mixture.
(iii) Name the type of reaction in which $\mathbf{P}$ forms $\mathbf{Q}$. [1]
(c) Platinum forms a complex ion with the formula $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$. In this complex ion the carbon atom of each $\mathrm{CN}^{-}$ligand bonds to the platinum ion. This complex shows stereoisomerism.
(i) There are only two isomers of this complex.
Draw structures of these two isomers in the boxes below.
(ii) Describe the geometry of $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$.[1]
(iii) Name the type of stereoisomerism shown by $\left[\mathrm{Pt}(\mathrm{CN})_2 \mathrm{Cl}_2\right]^{2-}$.[1] [Total: 9]
▶️Answer/Explanation
Ans:
a)(i) (a species) that donates one lone pair [1] to form a dative / coordinate to a central metal atom / metal ion [1]
$6(\mathrm{a})$ (ii) $\quad\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}[1]$
(b)(i) $
\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}+2 \mathrm{CN}^{-} \rightarrow\left[\mathrm{Ag}(\mathrm{CN})_2\right]^{-}+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}[1]
$
OR
$
\left.\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]^{3-}+2 \mathrm{NaCN} \rightarrow\left[\mathrm{Ag}(\mathrm{CN})_2\right]^{-}+\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3+\mathrm{S}_2 \mathrm{O}_3{ }^{2-}
$
(b)(ii) Q is more stable / has a larger Kstab than P [1]
(b)(iii) ligand exchange / displacement / substitution
(c)(i)
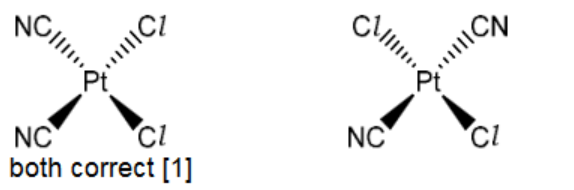
c)(ii) square planar [1] 1
6(c)(iii) cis-trans OR geometric(al) [1]
Question
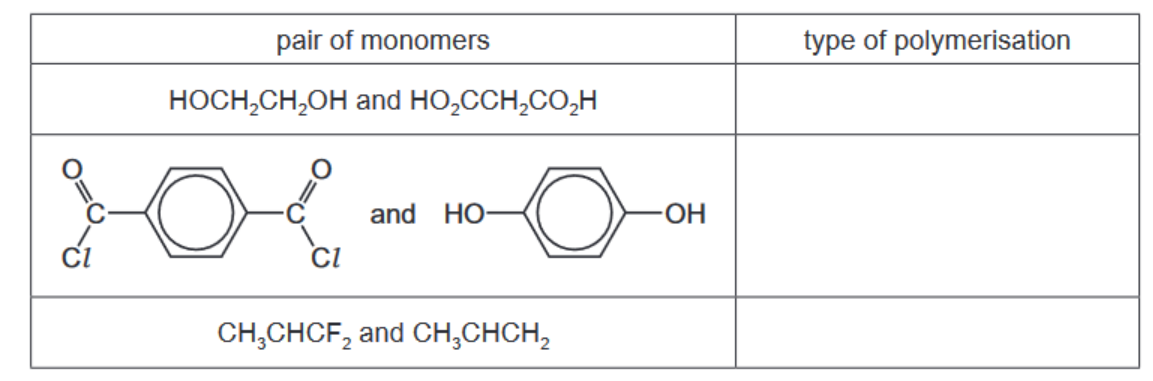
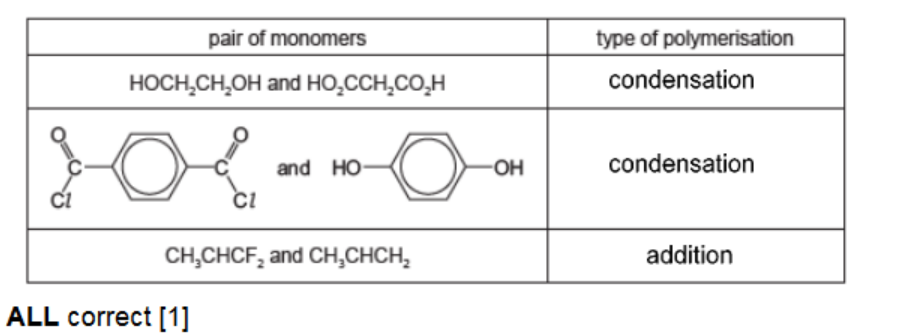
(a) The table shows three pairs of monomers that are capable of polymerisation. Complete the table by identifying each type of polymerisation.
(b) 2-aminopropanoic acid, $\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{NH}_2\right) \mathrm{CO}_2 \mathrm{H}$, can polymerise under suitable conditions. No other monomer is involved in this reaction.
(i) Draw a section of the polymer chain formed including three monomer residues. Clearly identify one repeat unit on your diagram.[3]
(ii) 2-aminopropanoic acid, $\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{NH}_2\right) \mathrm{CO}_2 \mathrm{H}$, exists as two stereoisomers.
Draw three-dimensional diagrams to show the two stereoisomers of 2-aminopropanoic acid. State the type of stereoisomerism shown.
type of stereoisomerism[5]
c) The skeletal formula of compound W is shown
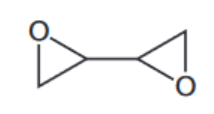
When $\mathbf{W}$ is mixed with a second compound, called a hardener, a polymerisation reaction occurs, producing a non-solvent-based adhesive.
(i) Give the name of this type of non-solvent-based adhesive.[1]
(ii) The hardener is a diamine. A diamine has an alkyl chain with two amine groups which are not bonded to the same carbon atom.
Draw the structural formula of a compound that would make a suitable hardener.[1][Total: 8]
▶️Answer/Explanation
Ans:
(a)
(b)(i)
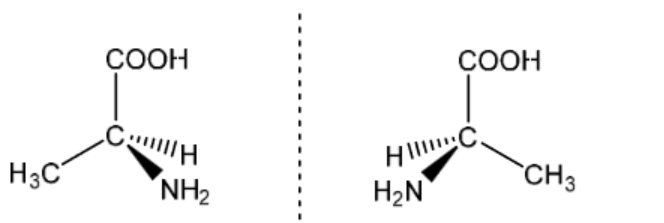
3D, tetrahedral, both isomers of 2-aminopropanoic acid [1]
optical [1]
(c)(i) epoxy resin [1] ALLOW Super Glues
(c)(ii) compound with two amine groups per molecule, amine groups must not be on the same carbon atom [1] e.g. $\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2$