Question
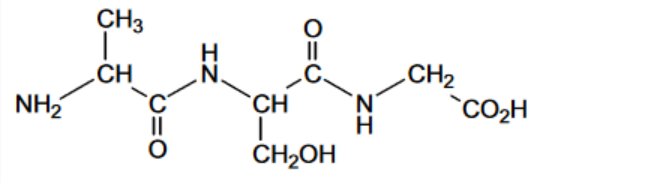
(a) (i) Use the Data Booklet to draw the structure of the tripeptide Ala-Ser-Gly showing its peptide bonds in full. Ala-Ser-Gly [2]
(ii) Calculate the relative molecular mass, $M_{\Gamma}$, of Ala-Ser-Gly.
$M_{\mathrm{r}}=$[1]
(b) Electrophoresis can be used to separate mixtures of amino acids and peptides.
A mixture of the tripeptide Ala-Ser-Gly and its three constituent amino acids was subjected to electrophoresis in a buffer at pH 11.
(i) Draw the structure of serine at pH 11. [1]
At the end of the experiment the following results were seen
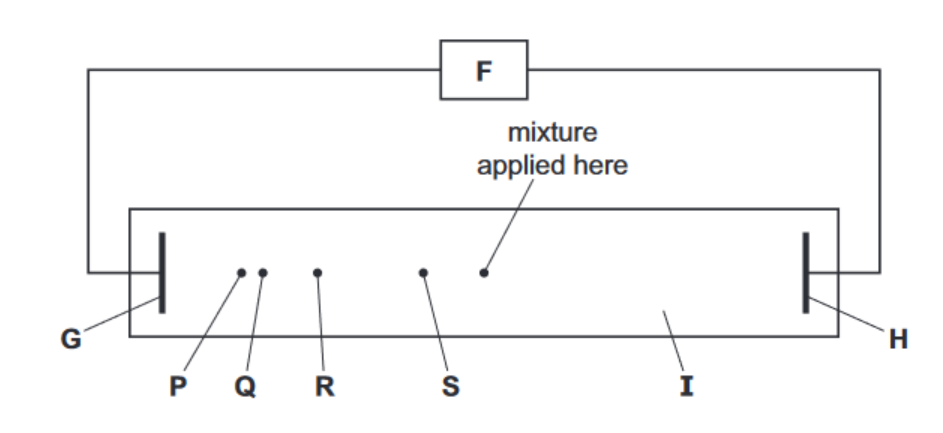
(ii) Identify the components labelled F–I in the above diagram.
F ………………………………………………………………………………………………………………………..
G ………………………………………………………………………………………………………………………..
H ………………………………………………………………………………………………………………………..
I …………………………………………………………………………………………………………………………
[4]
(iii) Suggest the identities of the species responsible for
spot P, ………………………………………………………………………………………………………………..
spot S. ………………………………………………………………………………………………………………..
Explain your answers.
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………… [3]
(c) (i) State the reagents and conditions needed for converting the tripeptide into its three constituent amino acids
ii) Name the type of reaction in (i).
……………………………………………………………………………………………………………………… [1] [Total: 13]
▶️Answer/Explanation
Ans:
(a) (i)
(ii) $M_{\mathrm{r}}=233$
(b) (i) $\mathrm{NH}_2 \mathrm{CH}\left(\mathrm{CH}_2 \mathrm{OH}\right) \mathrm{CO}_2^{-}$
(ii) F is a DC power supply
$\mathbf{G}$ is the anode OR positive electrode
$I$ is the cathode OR negative electrode
$\mathbf{H}$ is filter paper (OR gel) soaked in buffer solution
(iii) $\mathbf{P}$ is $\mathrm{NH}_2 \mathrm{CH}_2 \mathrm{CO}_2^{-}$or $\mathrm{NH}_2 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$ or glycine $\mathbf{S}$ is [ala-ser-gly] ${ }^{(-)}$ glycine is the smallest, so travels fastest; tripeptide is the largest, so travels slowest
(c) (i) heat with $\mathrm{H}_3 \mathrm{O}^{+} \mathrm{OR}$ heat with $\mathrm{OH}^{-}$(aq)
(ii) hydrolysis
Question
Procaine is used as an anaesthetic in medicine. It can be synthesised from methylbenzene in five steps as shown in Fig. 7.1.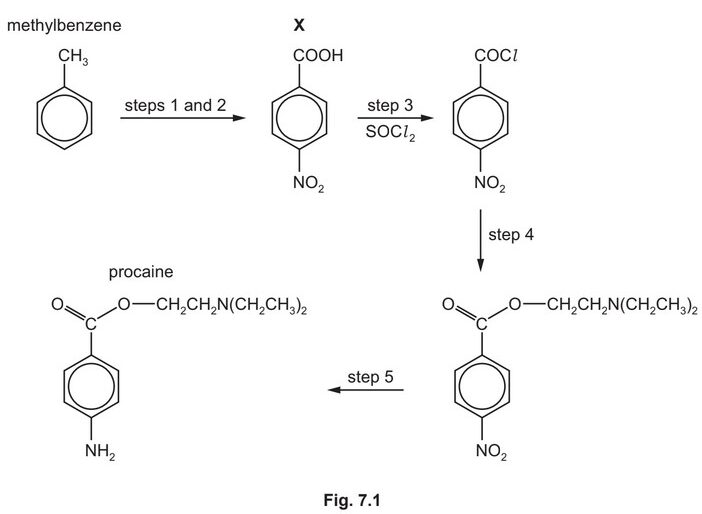
(a) (i) Name all the functional groups present in procaine.
(ii) A molecule of procaine has 13 carbon atoms.
State the number of carbon atoms that are sp, \(sp^2\) and \(sp^3\) hybridised in procaine.
sp carbons = ………………… \(sp^2\) carbons = ………………… \(sp^3\) carbons = …………………
(b) The proton (\(^1H\)) NMR spectrum of procaine dissolved in \(D_2O\) is recorded.
Predict the number of peaks observed.
(c) State why procaine can act as a base.
(d) Compound X can be synthesised in two steps from methylbenzene.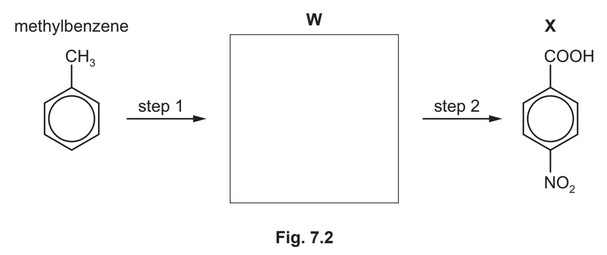
(i) Draw the structure of compound W in the box provided. [1]
(ii) State the reagents and conditions for step 1 and step 2.
step 1 ………………………………………………………………………………………………………………….
step 2 ………………………………………………………………………………………………………………….
(e) Procaine is synthesised in three steps from X.
Suggest the reagents and conditions for step 4 and for step 5 in Fig. 7.1.
step 4 ………………………………………………………………………………………………………………………..
step 5 ………………………………………………………………………………………………………………………..
(f) (i) Explain what is meant by partition coefficient, \(K_{pc}\).
(ii) The partition coefficient of procaine between octan-1-ol and water is 1.77.
Octan-1-ol and water are immiscible. A solution containing 0.500g of procaine in 75.0cm3 of water is shaken with 50.0\(cm^3\) of octan-1-ol.
Calculate the mass of procaine that is extracted into the octan-1-ol.
mass of procaine extracted = ………………………… g
Answer/Explanation
Answer:
(a) (i) phenylamine AND amine AND ester
(ii) sp carbons = 0, \(sp^2\) carbons = 7, \(sp^3\) carbons = 6
(b) 6
(c) lone pair on the N can accept a proton
(d) (i) 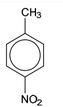
(ii) step 1 M1 concentrated \(HNO_3\) and \(H_2SO_4\)
step 2 M2 hot (alkaline) KMnO4 (followed by addition of \(H^+\))
(e) step 4 M1 \(HOCH_2CH_2N(CH_2CH_3)_2\)
step 5 \(M_2\) Sn AND HCl
M3 concentrated (HCl) AND heat / reflux
(f) (i) M1 ratio of the concentration of a solute in two solvents
M2 at equilibrium
(ii) M1 \(K_{pc} = [procaine]_{oct} / [procaine]_{water}\)
1.77 = (x / 50)/(0.5 – x / 75)
M2 1.77 = 1.5x / 0.5 – x
0.885 –1.77x = 1.5x
x = 0.271 g min 2sf