Question
Benzene, $\mathrm{C}_6 \mathrm{H}_6$, can be obtained from crude oil.
(a) Benzene reacts with bromine, in the presence of a suitable catalyst, forming bromobenzene as one product.
(i) Give the name or formula of the other product of this reaction. [1]
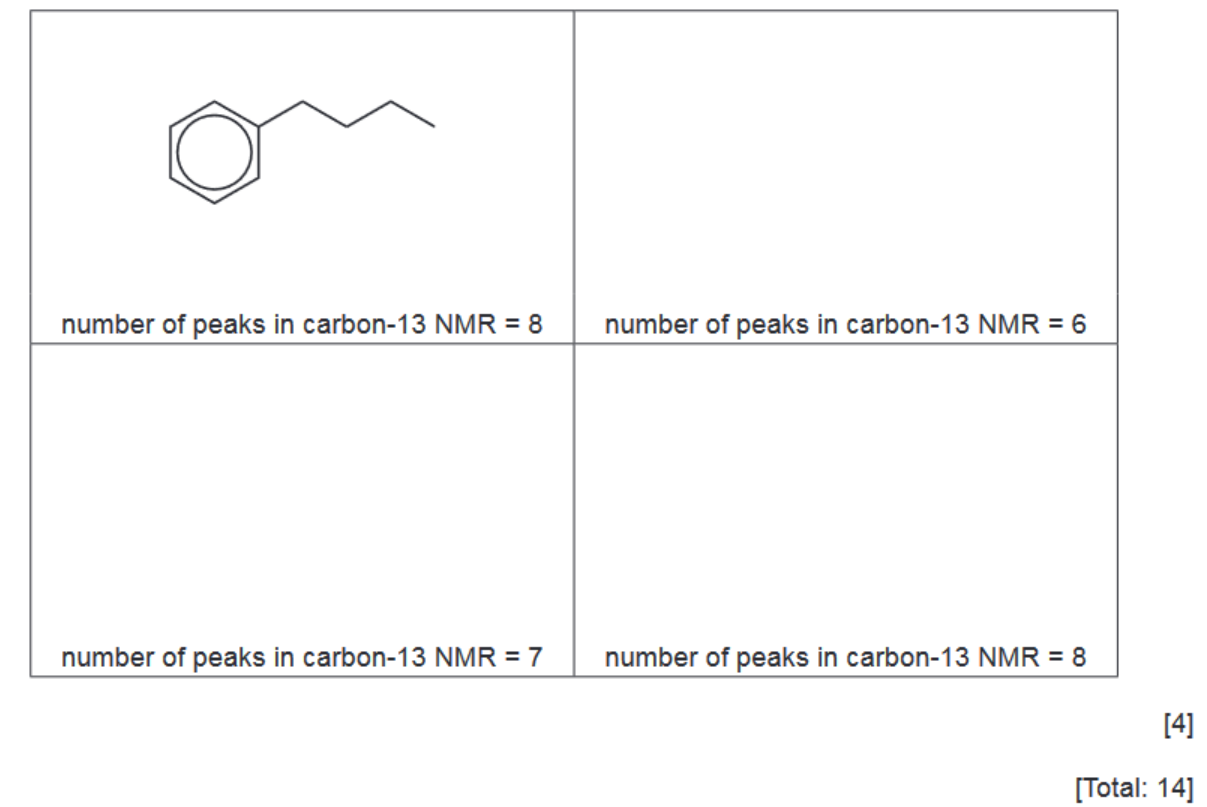
(ii) In the presence of the catalyst, bromine can be considered to form the electrophile $\mathrm{Br}^{+}$.
Complete the mechanism by which benzene reacts with $\mathrm{Br}^{+}$, using curly arrows to show the movement of electron pairs.
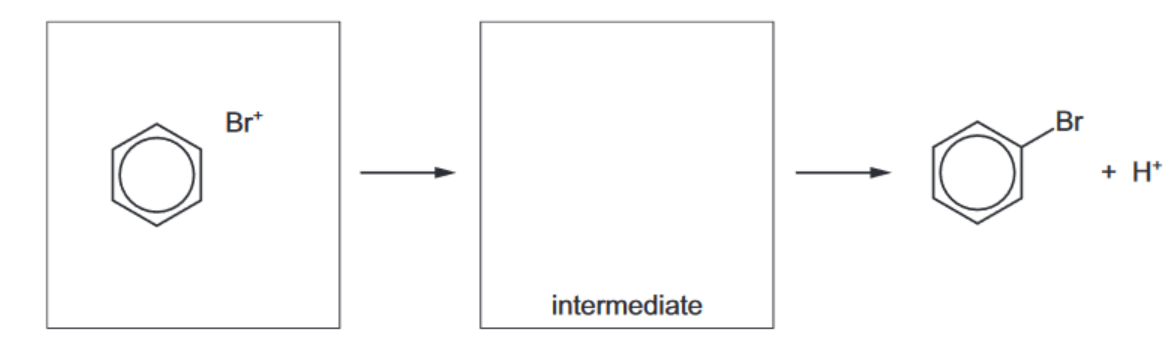
(iii) Name this mechanism. ……………………………………………………………………………………………………………………… [1]
(b) Benzene can be used as a starting material in the synthesis of cyclohexylmethanol, $\mathrm{C}_6 \mathrm{H}_{11} \mathrm{CH}_2 \mathrm{OH}$, as outlined below.
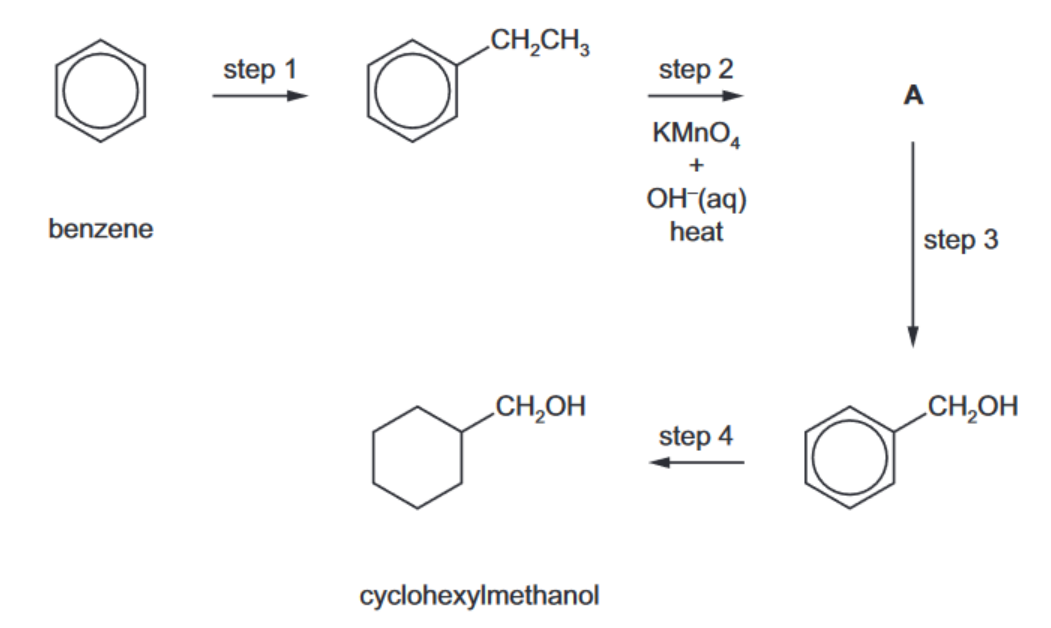
i) Identify a suitable reagent and a suitable catalyst for step 1.
reagent ……………………………………………………………………………………………………………….
catalyst ………………………………………………………………………………………………………………. [2]
(ii) Draw the structure of A
(iii) Identify suitable reagents for steps 3 and 4 .
step 3
$\operatorname{step} 4$[2]
(iv) Deduce the number of peaks in the carbon-13 NMR spectrum of cyclohexylmethanol.[1] [Total: 10]
▶️Answer/Explanation
Ans:
8(a)(i) HBr / hydrogen bromide [1]
a)(ii)
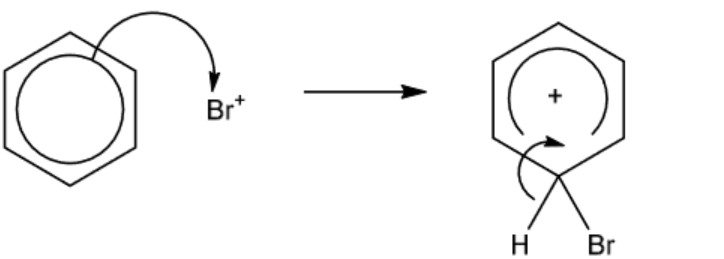
M1 curly arrow to $\mathrm{Br}^{+}$AND curly arrow from $\mathrm{C}-\mathrm{H}$ bond as shown [1] M2 correct intermediate [1]
(b)(i) reagent: chloroethane / bromoethane / iodoethane OR formula [1] catalyst: $\mathrm{FeCl}_3 / \mathrm{AlC}_3$ etc. [1]
(b)(ii)
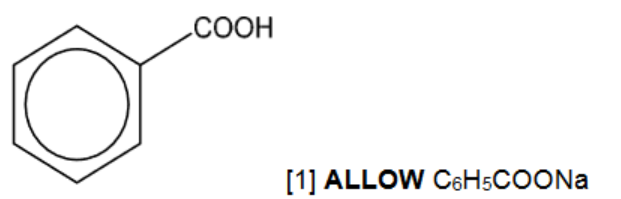
(b)(iii) step $3=\mathrm{LiAlH}_4[1]$
step $4=\operatorname{Pt}$ AND $\mathrm{H}_2[1]$
8(b)(iv) 5 / five [1]
Question
(a) Benzene reacts with bromine in the presence of an aluminium bromide catalyst, $\mathrm{AlBr}_3$, to form bromobenzene. This is a substitution reaction. No addition reaction takes place.
(i) Explain why no addition reaction takes place. [1]
$\mathrm{AlBr}_3$ reacts with bromine to generate an electrophile, $\mathrm{Br}^{+}$.
(ii) Draw the mechanism of the reaction between benzene and $\mathrm{Br}^{+}$ions. Include all relevant arrows and charges.[3]
(iii) Write an equation to show how the $\mathrm{AlBr}_3$ catalyst is reformed.[1]
(b) Suggest why bromination of phenol occurs more readily than bromination of benzene.[2]
(c) (i) There are four different carbocations with the same formula, $\mathrm{C}_4 \mathrm{H}_9^{+}$. One structure is given in the table.
Suggest the structural formulae of the three other carbocations.
 [3]
[3]
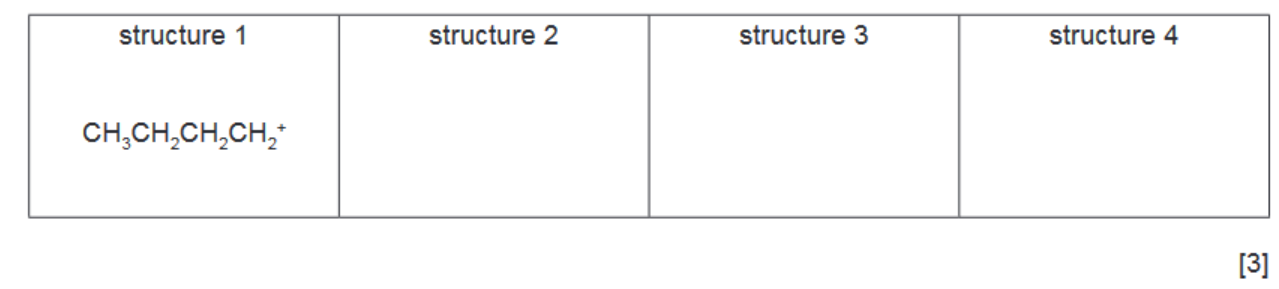
(ii) Benzene reacts with each of these carbocations in separate Friedel-Crafts alkylation reactions.
In each reaction an organic compound with formula $\mathrm{C}_{10} \mathrm{H}_{14}$ is formed. The number of peaks observed in the carbon-13 NMR spectrum of each compound is given.
Suggest the structures for the three other compounds.
▶️Answer/Explanation
Ans:
(a)(i) The substitution product is stabilised by delocalisation of (6)π-electrons
OR
The addition product is not stabilised by delocalisation of (6)π-electrons [1]
(a)(ii)
- first curly arrow
- intermediate
- $2^{\text {nd }}$ curly arrow, product and $\mathrm{H}^{+}$formed / lost
$5(\mathrm{a})$ (iii) $\quad \mathrm{AlBr}_4^{-}+\mathrm{H}^{+} \rightarrow \mathrm{AlBr}_3+\mathrm{HBr}$
5(b) lone pair of oxygen is delocalised into the ring
any one from:
• phenol has a higher electron density in the ring
• phenol can polarise/induce a dipole in $ Br_{2}$
5(c)(i) $\quad \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}^{+} \mathrm{CH}_3 \quad\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2{ }^{+} \quad\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+} \quad$ Each correct structure $=1$ mark
(c)(ii)
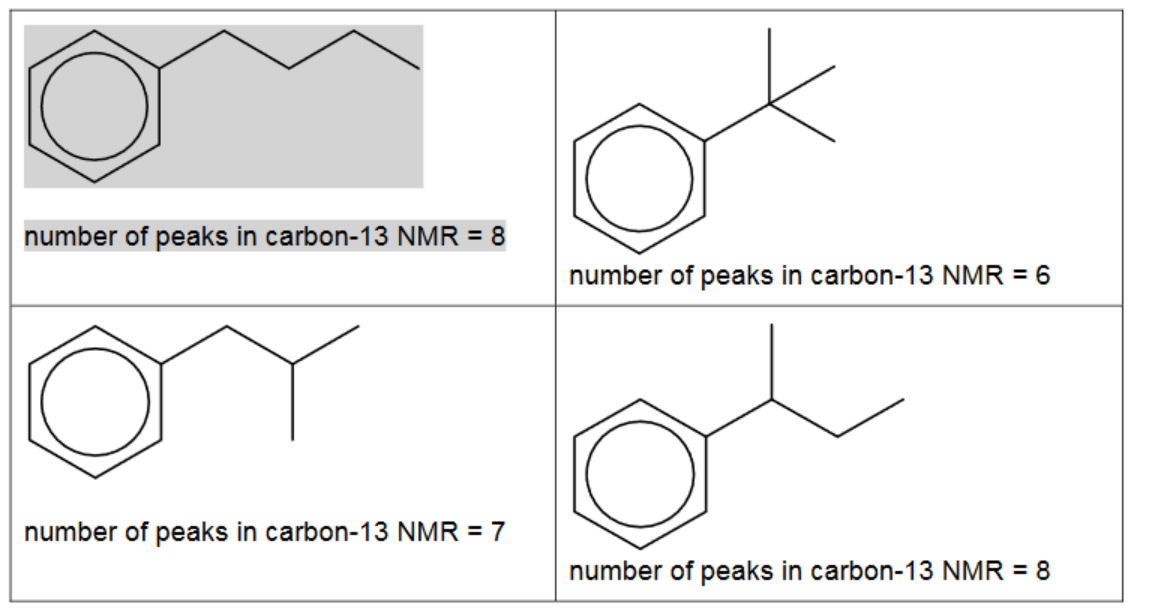
Two correct organic products = 1 mark
three correct organic products = 2 marks
all products linked correctly to NMR = 2 marks