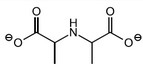
Question
(a) Describe what is meant by a racemic mixture.
(b) Asparagine is an amino acid that contains a chiral carbon atom and displays stereoisomerism.
Separate samples of asparagine are dissolved in \(CDCl_3\) and analysed using carbon-13 and proton (\(^1H\)) NMR spectroscopy.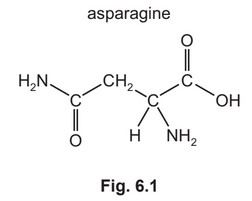
Predict the number of peaks seen in the carbon-13 and proton (\(^1H\)) NMR spectra of asparagine.
(c) The isoelectric point of asparagine, asn, is at pH 5.4.
(i) Describe the meaning of the term isoelectric point.
(ii) Draw the structure of asparagine at pH 1.0.
(d) Asparagine can polymerise to form poly(asparagine).
Draw the structure of poly(asparagine), showing two repeat units. The peptide linkage should be shown displayed.
(e) The isoelectric point of lysine, lys, is at pH 9.8.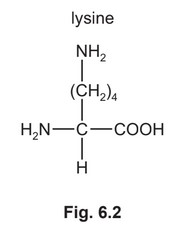
A mixture of the dipeptide lys-asn and its two constituent amino acids, asparagine and lysine, is analysed by electrophoresis using a buffer at pH 5.0. The results obtained are shown in
Fig. 6.3.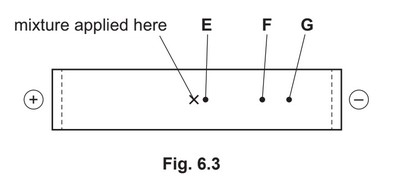
Suggest identities for the species responsible for spots E, F and G. Explain your answers.
(f) Thin-layer and gas-liquid chromatography can be used to analyse mixtures of substances.
Each type of chromatography makes use of a stationary phase and a mobile phase.
(i) Complete Table 6.1 with an example of each of these.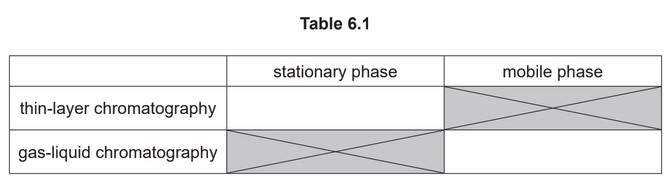
(ii) An unknown amino acid is analysed using thin-layer chromatography. Two chromatographs
of the unknown amino acid and four reference amino acids, P, Q, R and S, are obtained
using two different solvents.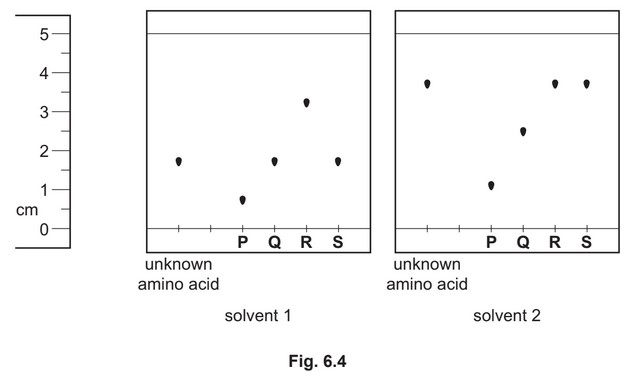
Identify the unknown amino acid. Justify your answer.
(g) A mixture containing three organic compounds is analysed by gas chromatography and mass spectrometry. The gas chromatogram is shown.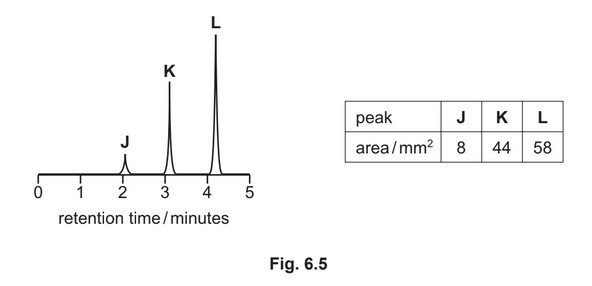
The area underneath each peak is proportional to the mass of the respective compound in the
mixture.
The concentration of K in the mixture is \(5.52 × 10^{–2} gdm^{–3}\).
Calculate the concentration, in moldm–3, of compound L in the mixture.
[\(M_r\): L, 116]
concentration of L = ………………………… \(moldm^{–3}\)
Answer/Explanation
Answer:
(a) a mixture containing equal amounts of each optical isomer
(b) 
(c) (i) the pH at which an amino acid exists as a zwitterion
OR
the pH at which an amino acid has no overall charge
(ii) 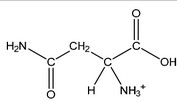
(d) 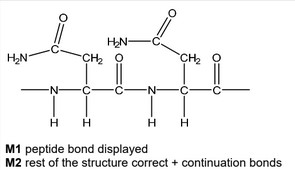
(e) 
M1 table correctly completed
M2 Lys and Lys-Asn are positively charged OR Asn is (nearly) uncharged
M3 LysAsn has the highest \(M_r\)
(f) (i) aluminum oxide / silica (on solid support) AND inert gas / named inert gas e.g. \(N_2\)
(ii) S AND
\(R_f\) is the same as the unknown amino acid in both solvents
(g) mass of L = 58 / 44 * 5.52 \times 10^{–2} = 7.28
\times 10^{–2}\) g
conc. of L = 7.28 \times 10^{–2} / 116 = 6.27 \times 10^{–4} (mol dm^{–3}) min 2sf\)
Question
2-Chloropropanoic acid, \(CH_3CHClCOOH\), is used in many chemical syntheses.
(a) (i) An equilibrium is set up when \(CH_3CHClCOOH\) is added to water.
Write the equation for this equilibrium.
(ii) 0.150mol of \(CH_3CHClCOOH\) dissolves in \(250cm^3\) of distilled water to produce a solution of pH 1.51.
Calculate the pKa of \(CH_3CHClCOOH\).
\(pK_a\) = …………………………
(iii) An equal concentration of aqueous propanoic acid has pH 2.55.
Explain the difference in the pH of solutions of equal concentration of \(CH_3CHClCOOH\) and propanoic acid.
(b) When \(CH_3CHClCOOH\) reacts with aqueous \(NH_3\), alanine forms.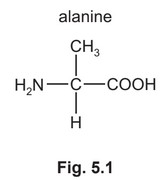
Alanine is an amino acid. Its isoelectric point is 6.1.
(i) State what is meant by isoelectric point.
(ii) Give the structural formula of alanine at pH 2.
(iii) Alanine exists as a pair of optical isomers. The structure of one optical isomer is shown in Fig. 5.2.
Draw the three-dimensional structure of the other optical isomer of alanine.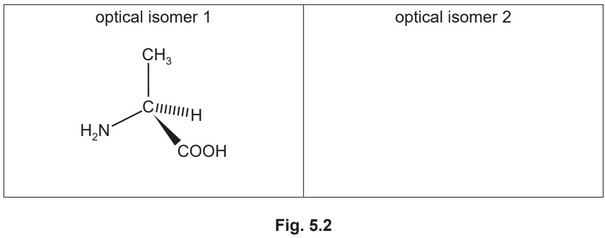
(iv) Polymer C forms from the reaction between alanine and 4-aminobutanoic acid,
\(H_2N(CH_2)_3COOH\).
Draw a repeat unit of C. The functional group formed should be displayed.
(v) State the type of polymerisation shown in (b)(iv).
(vi) Scientists are investigating C as a replacement for poly(propene) in packaging.
Suggest an advantage of using C instead of poly(propene).
(c) A student studies the reaction of \(CH_3CHClCOOH\) with aqueous \(NH_3\) to determine the reaction mechanism.
The student finds that when \(CH_3CHClCOOH\) and \(NH_3\) are added in a 1:1 stoichiometric ratio,
the conjugate acid and base of the reactants are quickly formed.
reaction 1 \(CH_3CHClCOOH + NH_3 → CH_3CHClCOO^–
+ NH_4^+\)
(i) Identify the conjugate acid–base pairs in reaction 1.
conjugate acid–base pair I ………………………………………. and ……………………………………..
conjugate acid–base pair II ……………………………………… and ……………………………………..
In an excess of \(NH_3\), \(CH_3CHClCOO^–\) undergoes a nucleophilic substitution reaction.
reaction 2 \(CH_3CHClCOO^– + NH_3 → CH_3CH(NH_2)COO^– + H^+ + Cl^–\)
A student investigates the rate of reaction 2. The student mixes \(CH_3CHClCOO^–\) with a large excess of \(NH_3\). The graph in Fig. 5.3 shows the results obtained.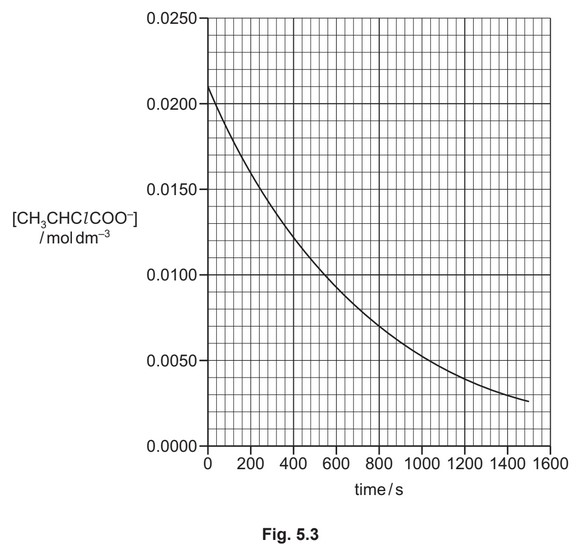
(ii) Use the graph in Fig. 5.3 to show that reaction 2 is first order with respect to \([CH_3CHClCOO^–]\).
(iii) Explain why a large excess of \(NH_3\) needs to be used in order to obtain the results in
Fig. 5.3.
(iv) The student measures the effect of changing the concentration of \(NH_3\) on the rate of
reaction 2. Table 5.1 shows the results obtained.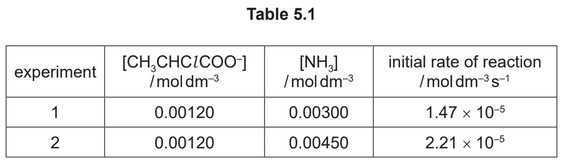
Use the information in Table 5.1 and in (c)(ii) to determine whether the nucleophilic
substitution reaction proceeds via an \(S_N1\) or an \(S_N2\) mechanism.
Explain your answer.
(v) Describe the effect of an increase in temperature on the rate of reaction of \(CH_3CHClCOO^–\) and \(NH_3\).
Explain your answer.
(vi) When an excess of \(CH_3CHClCOO^–\) is used, further substitution reactions occur. One
product has the formula \(C_6H_9NO_4^{2–}\).
Suggest the structure of \(C_6H_9NO_4^{2-}\)
Answer/Explanation
Answer:
(a) (i) \(CH_3CHClCOOH + H_2O ⇌ CH_3CHClCOO^– + H_3O^+\)
OR \(CH_3CHClCOOH ⇌ CH_3CHClCOO^– + H^+\)
(ii) \(M1: [H^+] = 10^{–1.51} = 0.0309 (mol dm^{– 3})\)
\(M2: K_a = 0.0309^2/0.60 = 1.592 × 10^{–3} ecf\)
\(pK_a = –log 1.592 × 10^{–3} = 2.80 ecf\)
(iii) \(CH_3CHClCOOH\) is a stronger acid (than propanoic acid, owing to higher \([H^+]\) [1]
because electron-withdrawing effect of Cl (substituent) AND weakens O—H / carboxylate anion stabilised [1]
(b) (i) pH at which a molecule has no overall charge / is neutral OR pH at which it exists as a zwitterion / dipolar ion
(ii) \(CH_3CH(N^+H_3)COOH\)
(iii) 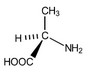
(iv) 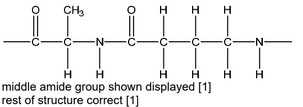
(v) condensation
(vi) C is biodegradable / easily hydrolysed
(c) (i) pair I = \(CH_3CHClCOOH\) (acid) and \(CH_3CHClCOO^–\) (c.base)
pair II = \(NH_3\) (base) and \(NH_4^+\) (c.acid)
(ii) evidence on graph / paper of one half-life (use of data) / \(t_{1/2} = 500 s [1]\)
constant half-life (= first order) [1]
(iii) so that \([NH_3]\) is (effectively) constant AND doesn’t affect the rate / zero order
(iv) M1: when \([NH_3]\) increases ×1.5, rate increase ×1.5 AND first order (w.r.t. \([NH_3])\) / rate is proportional to \([NH_3]\)
M2: \(SN_2\) DEP on M1
(because rate is first order w.r.t. to [2-chloropropanoate] and \([NH_3])\)
(v) greater proportion of particles have
\(E⩾E_A\)
frequency of (effective) collisions increases AND rate increases
OR rate of collisions increases AND rate increases [1]
(vi)