Question
(a) Define the term electron affinity.
(b) Write an equation for the process corresponding to the second ionisation energy of calcium.
Include state symbols.
Some data relating to calcium and oxygen are listed. Select relevant data from this list for your answers to parts (c), (d) and (e).
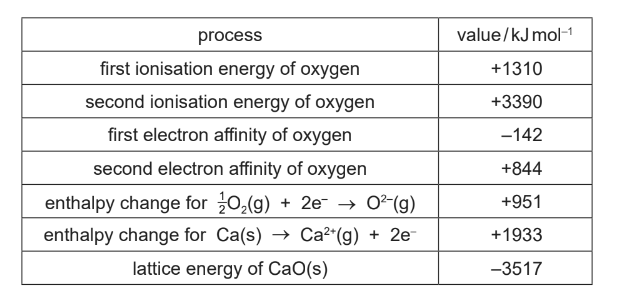
Show all your working.
(d) (i) Suggest why the first electron affinity of oxygen is negative.
(ii) Suggest why the second electron affinity of oxygen is positive.
(e) Calculate the enthalpy of formation of calcium oxide, CaO(s).
(f) The lattice energy of lithium fluoride, LiF(s), is –1022kJmol-1.
Identify the factor that causes the lattice energy of calcium oxide to be more exothermic than
that of lithium fluoride. Explain why this factor causes the difference in lattice energies.
Answer/Explanation
Answer (a) • enthalpy/energy change
• one mole of electrons gained
• by one mole of atoms
• gaseous (atoms)
(b) Ca+(g) → Ca2+(g) + e–
(c) M1: selecting correct data 951, 844, 142 only
M2: evaluation to give 249 (ΔHatom)
OR 2(951) = BE – 2(142) + 2(844)
M3: evaluation to 498 (2 × 249) ecf M2
951 =ΔHatom –142 + 844
ΔHatom = 249
BE = 498 (kJ mol–1)
(d)(i) attraction between nucleus / protons / nuclear charge
and electron
(d)(ii) repulsion between 1– ion / electrons of O–
and electron
(e) M1: selecting correct data 951, 1933, 3517 only (ignore signs)
M2: evaluation to give –633 (Δ Hf) ecf
ΔHf = 951 + 1933 – 3517 = –633 (kJ mol–1)
(f) ionic charge / charge density (of the ions)
greater (attractive) force between the ions
Question
Carbon monoxide gas, CO(g), and nitrogen gas, N2(g), are both diatomic molecules.
(a) The diagram shows the arrangement of outer electrons in a molecule of CO(g).
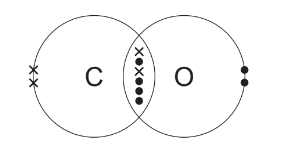
(i) State one similarity and one difference in the way the atoms in a carbon monoxide molecule are bonded together compared to the atoms in a nitrogen molecule.
(ii) The table states the electronegativity values of carbon, nitrogen and oxygen atoms.
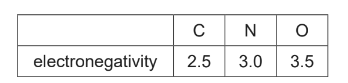
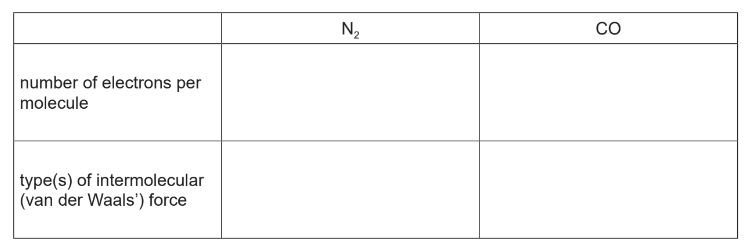
Use the electronegativity values and relevant details from the Data Booklet to complete the table below.
(b) N2(g) is less reactive than CO(g) even though N2(g) has a lower bond energy than CO(g). Suggest why CO(g) is more reactive than N2(g).
(c) Both carbon monoxide and nitrogen are gases at room temperature and pressure.
They both behave like ideal gases under certain conditions.
(i) State the two conditions necessary for these two gases to approach ideal gas behaviour.
(ii) Explain why N2(g) behaves more like an ideal gas than CO(g) does at 20.0°C and 101kPa.
(d) Calculate the amount, in mol, of pure nitrogen gas which occupies 100cm³ at 101kPa and 20.0°C. Use relevant information from the Data Booklet. Show your working.
Assume nitrogen behaves as an ideal gas.
Answer/Explanation
Answer: (a)(i) M1 both make triple (covalent) bond / 3 shared pairs of electrons 1
M2 one bond in CO is coordinate / dative covalent / formed by donating a pair of electrons from O (to C)
(a)(ii)
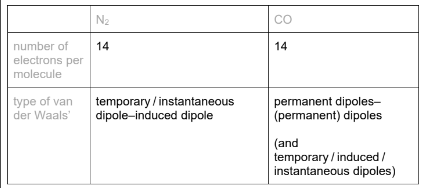
(b) CO / it is a polar molecule / it has a (permanent) dipole (but N2 is non-polar)
(c)(i) high temperature AND low pressure
(c)(ii) M1 CO is polar / has a permanent dipole OR N2 is non-pola
M2 IMF in CO are (more) significant / larger OR IMF in N2 are smaller / less significant
Alternative answer
M1 (Size of) N2 smaller than CO
OR volume of N2 molecules / particles smaller
Alternative answer
M2 volume of N2 molecules / particles is more negligible
ORA
(d) M1 correct conversion to consistent units
P = 101 000 V = 100 / 1 000 000 = (1 × 10-4) T = 293
M2 use of all values from M1 in correct relationship, n = PV / RT
M3 calculation = 4.15 × 10-3 mol