Question
Phosphorus, sulfur and chlorine can all react with oxygen to form oxides.
(a) Phosphorus reacts with an excess of oxygen to form phosphorus(V) oxide.
(i) Write an equation to show the reaction of phosphorus with excess oxygen.[1]
(ii) Describe the reaction of phosphorus(V) oxide with water.[2]
(iii) State the structure and bonding of solid phosphorus(V) oxide.[1]
(b) The two most common oxides of sulfur are $\mathrm{SO}_2$ and $\mathrm{SO}_3$.
When $\mathrm{SO}_2$ dissolves in water, a small proportion of it reacts with water to form a weak Brønsted-Lowry acid.
(i) Explain the meaning of the term weak Brønsted-Lowry acid.[2]
(ii) Write the equation for the reaction of $\mathrm{SO}_2$ with water.$[1]$
(iii) $\mathrm{SO}_2$ reacts with $\mathrm{NO}_2$ in the atmosphere to form $\mathrm{SO}_3$ and $\mathrm{NO}$.
$\mathrm{NO}$ is then oxidised in air to form $\mathrm{NO}_2$.
$
\begin{gathered}
\mathrm{SO}_2+\mathrm{NO}_2 \rightarrow \mathrm{SO}_3+\mathrm{NO} \\
2 \mathrm{NO}+\mathrm{O}_2 \rightarrow 2 \mathrm{NO}_2
\end{gathered}
$
State the role of $\mathrm{NO}_2$ in this two-stage process. [1]
(c) Emissions of $\mathrm{SO}_2$ from coal-fired power stations can be reduced by mixing the coal with powdered limestone.
Limestone is heated to form $\mathrm{CaO}$ in reaction 1. This then reacts with $\mathrm{SO}_2$ and $\mathrm{O}_2$ to form $\mathrm{CaSO}_4$ in reaction 2.
reaction 1: $\quad \mathrm{CaCO}_3(\mathrm{~s}) \rightarrow \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~s})$
reaction 2: $\mathrm{CaO}(\mathrm{s})+\mathrm{SO}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CaSO}_4(\mathrm{~s})$
(i) State the type of reaction occurring in reaction 1.[1]
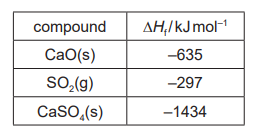
(ii) Use the data to calculate the enthalpy change of reaction 2 .
(d) Chlorine forms several oxides, including $\mathrm{Cl}_2 \mathrm{O}, \mathrm{ClO}_2$ and $\mathrm{Cl}_2 \mathrm{O}_6$.
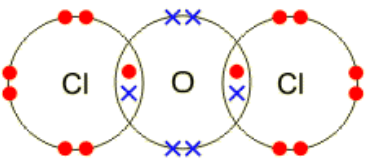
(i) Draw a ‘dot-and-cross’ diagram of $\mathrm{Cl}_2 \mathrm{O}$. Show outer-shell electrons only.[1]
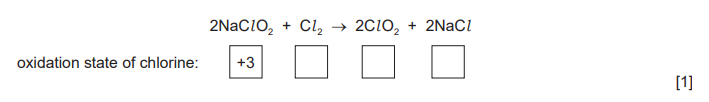
(ii) $\mathrm{ClO}_2$ can be prepared by reacting $\mathrm{NaClO}_2$ with $\mathrm{Cl}_2$.
Write the oxidation state of chlorine in each species in the boxes provided.
(iii) $\mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})$ is produced by the reaction of $\mathrm{ClO}_2(\mathrm{~g})$ with $\mathrm{O}_3(\mathrm{~g})$.
$
2 \mathrm{ClO}_2(\mathrm{~g})+2 \mathrm{O}_3(\mathrm{~g}) \rightleftharpoons \mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \quad \Delta H=-216 \mathrm{~kJ} \mathrm{~mol}^{-1}
$
The reaction takes place at $500 \mathrm{~K}$ and $100 \mathrm{kPa}$.
State and explain the effect on the yield of $\mathrm{Cl}_2 \mathrm{O}_6(\mathrm{~g})$ when the experiment is carried out:
- at $1000 \mathrm{~K}$ and $100 \mathrm{kPa}$
- at $500 \mathrm{~K}$ and $500 \mathrm{kPa}$. [4]
(e) Element E is a Period 5 element.
E reacts with oxygen to form an insoluble white oxide that has a melting point of $1910^{\circ} \mathrm{C}$. The oxide of $\mathbf{E}$ conducts electricity only when liquid.
$\mathrm{E}$ also reacts readily with $\mathrm{Cl}_2(\mathrm{~g})$ to form a white solid that reacts exothermically with water. The resulting solution reacts with aqueous silver nitrate to form a white precipitate that dissolves in dilute ammonia.
(i) Suggest the type of bonding shown by the oxide of E. Explain your answer.[2]
(ii) Suggest the type of bonding shown by the chloride of E. Explain your answer.[2] [Total: 21]
▶️Answer/Explanation
Ans:
$(\mathrm{a})(\mathrm{i}) \quad \mathrm{P}_4+5 \mathrm{O}_2 \rightarrow \mathrm{P}_4 \mathrm{O}_{10}$
(a)(ii) any two from:
• reacts vigorously
• solid disappears / colourless solution forms
• hydrolysis
• exothermic
• acid(ic) (solution)
• steamy / misty fumes
(a)(iii) Simple and covalent OR molecular and covalent
(b)(i) \text { M1: proton / } \mathrm{H}^{+} \text {donor }
M2: partially dissociates (in solution)
(b)(ii) $\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_3$
(b)(iii) (homogeneous) catalyst 1
(c)(i) thermal decomposition
(c)(ii) M1: $\Delta H_r=-1434-(-635+-297)$
M2: $=-502\left(\mathrm{~kJ} \mathrm{~mol}^{-1}\right)$
(d)(i)
(d)(ii)
![]()
(d)(iii) (at 1000 K and 100 kPa)
M1: (yield) decreases
M2: reaction is exothermic AND equilibrium moves left
(at 500 K and 500 kPa)
M3: (yield) increases
M4: fewer moles (of gas) on right-hand side AND equilibrium moves right
(e)(i) M1: ionic
M2: ions only able / free to move / free to conduct (when liquid / molten)
(e)(ii) M1: covalent
M2: hydrolysed (by water)