Question
The reducing agent $\mathrm{LiAlH}_4$ can be synthesised by reacting aluminium chloride with lithium hydride, $\mathrm{LiH}$.
(a) (i) At $200^{\circ} \mathrm{C}$, aluminium chloride exists as $\mathrm{Al}_2 \mathrm{Cl}_6(\mathrm{~g})$.
Draw the structure of $\mathrm{Al}_2 \mathrm{C} l_6(\mathrm{~g})$, showing fully any coordinate (dative covalent) bonds in the molecule.[2]
(ii) At $1000^{\circ} \mathrm{C}$, aluminium chloride exists as $\mathrm{AlCl} l_3(\mathrm{~g})$.
State the bond angle in $\mathrm{AlCl}_3(\mathrm{~g})$.
$\circ[1]$
(iii) Lithium hydride contains the ions $\mathrm{Li}^{+}$and $\mathrm{H}^{-}$.
State the electronic configuration of these two ions.
$\mathrm{Li}^{+}$
$\mathrm{H}^{-}$[1]
(iv) $\mathrm{LiAlH}_4$ decomposes slowly to form $\mathrm{LiAl}(\mathrm{s})$ and $\mathrm{H}_2(\mathrm{~g})$.
$
\mathrm{LiAlH}_4(\mathrm{~s}) \rightarrow \mathrm{LiAl}(\mathrm{s})+2 \mathrm{H}_2(\mathrm{~g})
$
LiAl(s) shows metallic bonding.Describe metallic bonding [1]
(b) $\mathrm{LiAlH}_4$ cannot be used in aqueous solution because it reacts with water to produce $\mathrm{LiOH}(\mathrm{aq})$, $\mathrm{H}_2(\mathrm{~g})$ and a white precipitate which is soluble in excess sodium hydroxide.
Identify the white precipitate. [1]
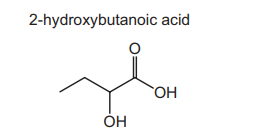
(c) Two students try to prepare 2-hydroxybutanoic acid in the laboratory.
Both students oxidise butane-1,2-diol to form $\mathbf{P}$ in reaction 1 . One student then reduces $\mathbf{P}$ using $\mathrm{LiAlH}_4$. $\mathbf{Q}$ is formed. The other student reduces $\mathbf{P}$ using $\mathrm{NaBH}_4$. $\mathbf{R}$ is formed.
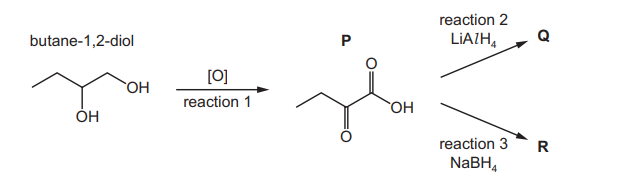
(i) State the reagents and conditions required for reaction 1.[2]
(ii) Only one of the students successfully prepares 2‑hydroxybutanoic acid.
Identify which of Q or R is 2‑hydroxybutanoic acid and explain the difference between reactions 2 and 3.
A third student prepares 2‑hydroxybutanoic acid using propanal as the starting material. In
step 1 the student reacts propanal with a mixture of NaCN and HCN.
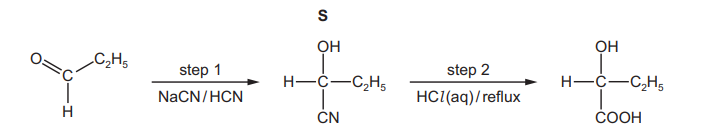
(iii) Draw the mechanism for the reaction of propanal with the mixture of NaCN and HCN to form S.
● Identify the ion that reacts with propanal.
● Draw the structure of the intermediate of the reaction.
● Include all charges, partial charges, lone pairs and curly arrows.

(iv) Complete the equation for the reaction in step 2, when S is heated under reflux with HCl(aq)
$
\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{CN}+ \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{COOH}+
$ [1]
(v) The infrared spectrum of an organic compound is shown. The organic compound is either S or 2‑hydroxybutanoic acid.
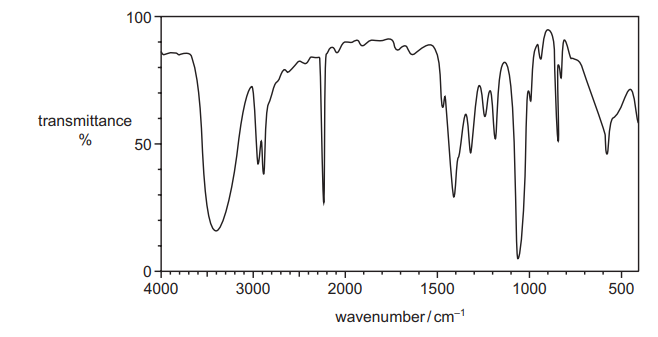
Deduce the identity of the compound. Give two reasons for your answer.
In your answer, identify any relevant absorptions above $1500 \mathrm{~cm}^{-1}$ in the spectrum and the bonds that correspond to these absorptions. [2] [Total: 17]
▶️Answer/Explanation
Ans:
(a)(i) M1: correct representation of $\mathrm{Al}_2 \mathrm{Cl} l_6$, dot and cross or line diagram
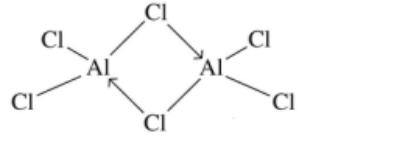
M2: TWO correct co-ordinate bonds identified
(a)(ii) 120
(a)(iii) $\mathrm{Li}^{+}$is $1 \mathrm{~s}^2 \quad \mathrm{H}^{-}$is $1 \mathrm{~s}^2$
(a)(iv) (Lattice of) cations / positive ions surrounded by delocalised electrons’
(b) $\mathrm{Al}(\mathrm{OH})_3 /$ aluminium hydroxide
(c)(i) M1: potassium dichromate[(VI)]
M2: acid(ified) AND (heat under) reflux
3(c)(ii) (M1: correct identity of
R and statement re: reaction 3 ONLY ketone reduced)
R (is 2-hydroxybutanoic acid) AND as (only) C=O / ketone reduced
(M2: correct explanation re: strength of reducing agents) $\mathrm{NaBH}_4$ cannot reduce the $\mathrm{COOH} /$ carboxylic acid OR
$\mathrm{LiAlH}_4$ can reduce the $\mathrm{COOH} /$ carboxylic acid
3(c)(iii)
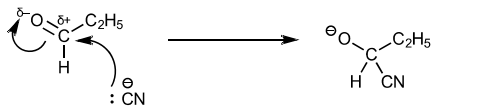
M1: Presence of :CN (if bonding shown, must be unambiguous triple bond)
M2: curly arrow from :CN lone pair to carbonyl carbon
M3: correct dipole AND curly arrow from double bond to oxygen
M4: correct intermediate drawn
(c)(iv) $\mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{CN}+\mathbf{H C l}+\mathbf{2} \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{CH}(\mathrm{OH}) \mathrm{COOH}+\mathbf{N H}_4 \mathbf{C l}$
(c)(v) Any two of three absorption references:
- absorption 2200-2250 $\left(\mathrm{cm}^{-1}\right)$ shows presence of $\mathrm{C} \equiv \mathrm{N}$
- lack of absorption at $1680-1730\left(\mathrm{~cm}^{-1}\right)$ shows lack of $C=O$
- lack of absorption at $2500-3000\left(\mathrm{~cm}^{-1}\right)$ shows lack of $\mathrm{RCO}_2-\mathrm{H} / \mathrm{O}-\mathrm{H}$ in $\mathrm{RCO}_2 \mathrm{H}$
Question
Iron pyrite, FeS2, has a yellow colour that makes it look like gold metal. The compound contains the
ions Fe2+ and S22–.
(a) (i) Give the full electronic configuration of Fe2+.
(ii) Calculate the oxidation number of sulfur in the S22– ion. Assume that each sulfur atom in the ion has the same oxidation number.
(b) Describe the metallic bonding in gold.
(c) Iron pyrite is often called fool’s gold because of its appearance. Impure samples of iron pyrite often contain a small amount of gold.
The gold can be obtained from impure iron pyrite. The impure iron pyrite is roasted in oxygen, to produce iron(III) oxide and sulfur dioxide. Gold does not react with oxygen.
(i) The sulfur dioxide produced during roasting would cause environmental consequences if released into the atmosphere.
State and explain one of these environmental consequences.
(ii) Complete the equation to show the roasting of iron pyrite in oxygen.
\(4FeS_{2} + ……….. \rightarrow 2Fe_{2}O_{3} + ………..\)
(iii) A sample of impure iron pyrite was roasted in oxygen. The composition of the mixture of
solid products is shown.
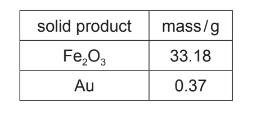
Calculate the mass of FeS2 present in the sample of impure iron pyrite.
Assume that all the FeS2 was converted to Fe2O3 during the roasting process.
(Mr : FeS2, 120.0; Fe2O3, 159.6)
(iv) Use your answer to (iii) to calculate the percentage by mass of gold in this sample of
impure iron pyrite. Assume that gold is the only impurity in this sample of impure iron pyrite.
Give your answer to two significant figures.
(If you were unable to calculate an answer to (iii), use 55.00g as the mass of FeS2 in this
calculation. This is not the correct answer.
Answer/Explanation
Answer: (a)(i) 1s2 2s2 2p6 3s2 3p6 3d6 (4s0)
(a)(ii) –1
(b) M1 attraction/hold
M2 positive ions / cations AND delocalised electrons (may be seen in a labelled diagram)
(c)(i) M1 acid rain
M2
• destroys / damages / weathers / erodes / buildings / statues
• kills/harms fish / coral / plants / crops / trees / deforestation
• leaches salts / ions (aluminium) from soil (into rivers / lakes)
• leaches away soil nutrients
• breathing difficulties
• lowers pH / increases acidity of soil / rivers / oceans / seas
(c)(ii) balanced equation with 11O2 and 8SO2
M1: O2 and SO2
M2: 11 and 8
(c)(iii) M1 is for process of calculating number of moles of Fe2O3
33.18 ÷ 159.6 (= 0.2079 mol)
M2 for correct use of stoichiometry and 120.0 with candidate’s M1
M2 (0.2079) × 4 / 2 × 120.0 = 49.89 (g)
(c)(iv) (0.37/(0.37+49.89)) = 0.74