Question:
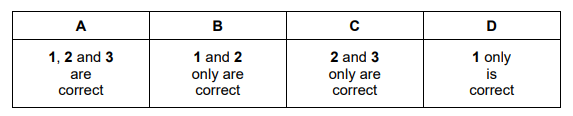
Which species can accept a lone pair of electrons to form a coordinate (dative covalent) bond?
1 \(BF_{3}\)
2 \(H^{+}\)
3 \(CH_{3}^{~+}\)
▶️Answer/Explanation
Ans:C
Question
Which statement about aluminium chloride is correct?
A Aluminium chloride has a much higher melting point than magnesium chloride due to the small size of the aluminium ion.
B Anhydrous aluminium chloride reacts vigorously with water to form a solution with a pH greater than 7.
C Each \(Al_2Cl_6\) molecule found in aluminium chloride vapour contains two coordinate bonds.
D The bonding between aluminium and chlorine is strongly ionic due to the large difference in electronegativity.
Answer/Explanation
Ans: C
Question
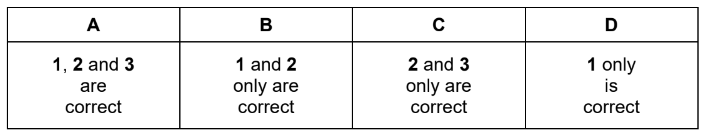
Which elements form a chloride in which both covalent bonding and coordinate (dative covalent) bonding are present?
1 Al
2 Si
3 Mg
Answer/Explanation
Answer D
Question
The eight species that follow all have covalent bonds.
In which pair do the species have different shapes from each other?
A BeCl2 and CO2
B CH4 and NH4+
C NH3 and BF3
D SCl2 and H2O
Answer/Explanation
Answer:
C
Question
Which types of bonding are present in ammonium carbonate, (NH4)2CO3?
1 ionic
2 covalent
3 co-ordinate (dative covalent)
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
A
Question
Which statement is correct?
A Ammonia reacts with alkalis to form the ammonium ion.
B Ammonium chloride contains ionic, covalent and co-ordinate bonds.
C The ammonium ion reacts with acids to produce ammonia.
D The bond angle in the ammonium ion is approximately 107°.
Answer/Explanation
Answer B
Question
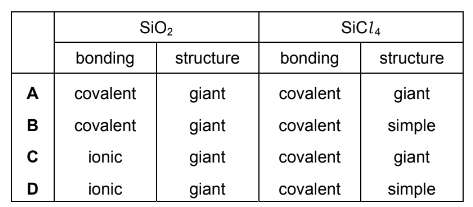
Which row describes the structure and bonding of SiO2 and SiCl4?
Answer/Explanation
Answer B
Question
The definitions of many chemical terms can be illustrated by chemical equations.
Which terms can be illustrated by an equation that includes the formation of a positive ion?
1 first ionisation energy
2 heterolytic fission of a covalent bond
3 enthalpy change of atomisation
The responses A to D should be selected on the basis of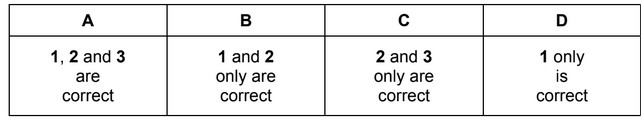
Answer/Explanation
Ans: B
Question
Which molecule contains a nitrogen atom with sp hybridised orbitals?
A CH3CH2NH2 B HNO3 C HCN D NH3
Answer/Explanation
Answer:
C
Question
In the sodium chloride lattice the number of chloride ions that surround each sodium ion is called the co-ordination number of the sodium ions.
What are the co-ordination numbers of the sodium ions and the chloride ions in the sodium chloride lattice?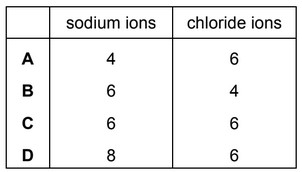
Answer/Explanation
Ans: C
Question
What is the correct number of bonds of each type in the Al2Cl6 molecule?
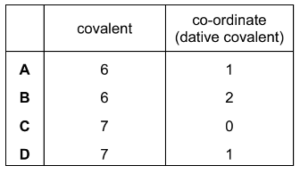
Answer/Explanation
Answer: B
Question
Some car paints contain small flakes of silica, \(SiO_{2}\).
In the structure of solid \(SiO_{2}\)
● each silicon atom is bonded to x oxygen atoms,
● each oxygen atom is bonded to y silicon atoms,
● each bond is a z type bond.
What is the correct combination of x, y and z in these statements?
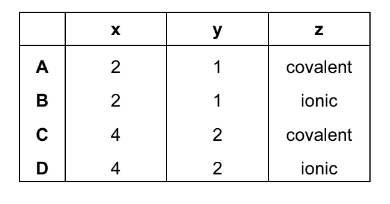
Answer/Explanation
Ans:C
Question
The table shows the physical properties of four substances. Which substance has a giant covalent structure?
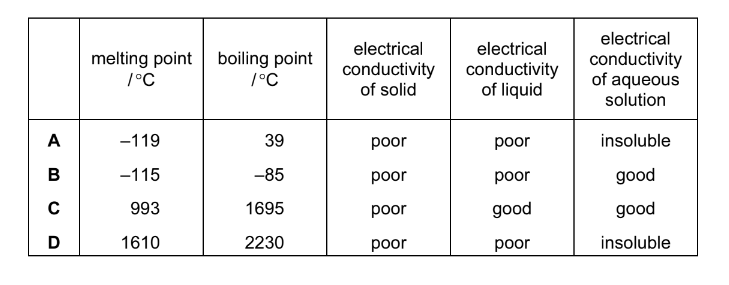
Answer/Explanation
Ans:D
Question
Which types of bonding are present in ammonium carbonate, \((NH_4)2CO_3\)?
1 ionic
2 covalent
3 co-ordinate (dative covalent)
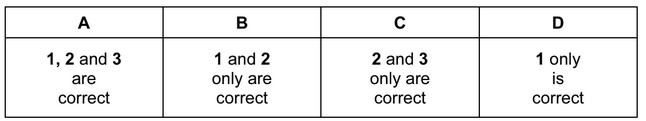
Answer/Explanation
Ans: A
