Question
Which molecules have no overall dipole moment?
1 boron trifluoride
2 methane
3 phosphorus pentafluoride
▶️Answer/Explanation
Ans:A
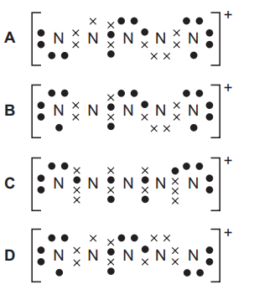
Question:
A stable ion \(N_{5}^{~+}\) has been produced by research chemists. Which structure is most likely to show the electron arrangement of this ion?
▶️Answer/Explanation
Ans:B
Question:
Which molecule does not have any 90° or 180° bond angles?
A \(C_{2}H_{6}\) B \(CO_{2}\) C \(PF_{5}\) D \(SF_{6}\)
▶️Answer/Explanation
A
Question
The characteristic smell of garlic is due to alliin.
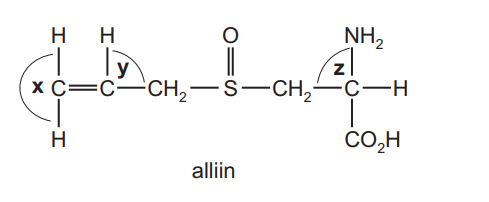
What are the approximate bond angles x, y and z in a molecule of alliin?
▶️Answer/Explanation
Ans:C
Question
Which series shows molecules in order of increasing bond angle?
A $\mathrm{CH}_4 \rightarrow \mathrm{BF}_3 \rightarrow \mathrm{NH}_3$
B $\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CO}_2 \rightarrow \mathrm{BF}_3$
C $\mathrm{NH}_3 \rightarrow \mathrm{CH}_4 \rightarrow \mathrm{CO}_2$
D $\mathrm{NH}_3 \rightarrow \mathrm{CH}_4 \rightarrow \mathrm{H}_2 \mathrm{O}$
▶️Answer/Explanation
Ans:C
Question
For which molecule is the dipole moment zero?
A \(CH_3Cl\) B \(CH_2Cl_2\) C \(CHCl_3\) D \(CCl_4\)
Answer/Explanation
Ans: D
Question
When ammonia, \(NH_3\), is dissolved in water, a small concentration of ammonium ions, \(NH_4^+\), is formed.
Which row is correct?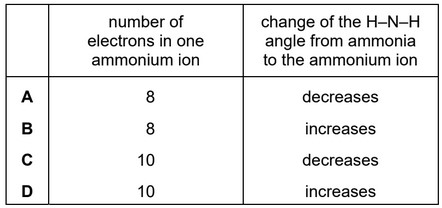
Answer/Explanation
Ans: D
Question
Phosphorus forms two chlorides. Phosphorus(III) chloride, PCl3 , is a covalent liquid.
Phosphorus(V) chloride is an ionic solid. One of the ions present is [PCl4]+.
What is the shape of the PCl3 molecule and the [PCl4]+ion?
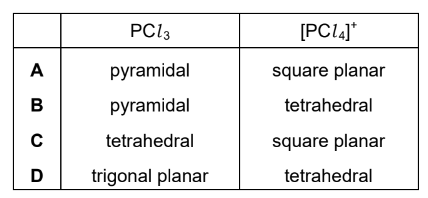
Answer/Explanation
Answer: B
Question
Which molecules contain at least one bond angle of 120 °?
1 C2H4
2 PF5
3 NCl3
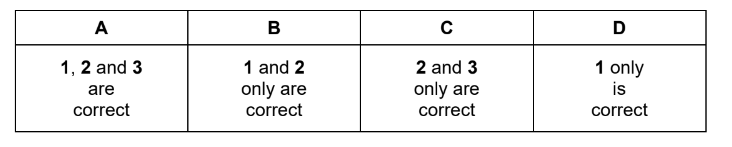
Answer/Explanation
Answer: B
Question
The eight species that follow all have covalent bonds.
In which pair do the species have different shapes from each other?
A BeCl2 and CO2
B CH4 and NH4+
C NH3 and BF3
D SCl2 and H2O
Answer/Explanation
Answer:
C
Question
Histidine is an amino acid.
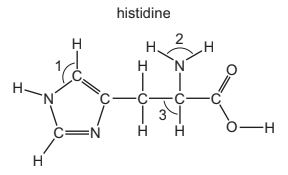
What are the approximate bond angles 1, 2, and 3?
Answer/Explanation
Answer:
B
Question
Which molecule or ion contains the smallest bond angle?
A C2H4 B CH3COCH3 C NH4+ D NH3
Answer/Explanation
Answer D
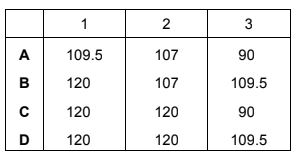
Question
Which molecules and ions have a bond angle of 120°?
1 BF3
2 CH3–
3 NH3
Answer/Explanation
Answer D
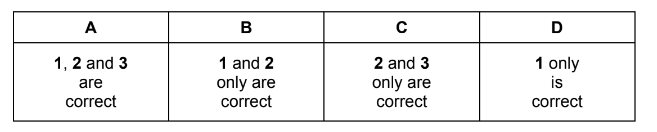
Question
What are the shapes of the molecules of water and boron trifluoride?
Answer/Explanation
Answer:
D
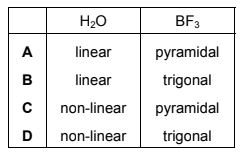
Question
Histamine is produced in the body to help fight infection. Its shape allows it to fit into receptors which expand blood vessels.
What are the bond angles x, y and z in histamine, from the smallest to the largest?
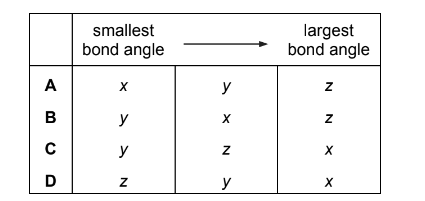
Answer/Explanation
Answer: A
Question
Urea is a product of animal metabolism. It can also be used as a fertiliser.
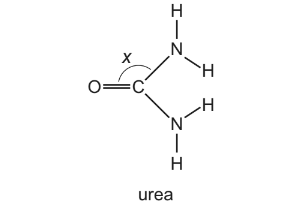
The diagram shows angle x in this molecule.
Which statements about the structure of urea are correct?
1 Angle x is approximately 120°.
2 The molecule has two π bonds.
3 The molecule has only three lone pairs of electrons.
The responses A to D should be selected on the basis of
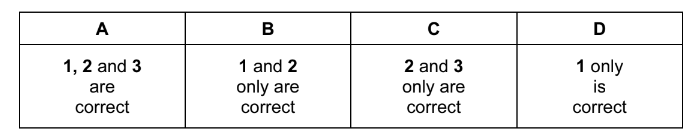
Answer/Explanation
Ans:D
Question
\(AlCl_{ 3}\) vapour forms molecules with formula\( Al _{2}Cl _{6 }\)as it is cooled.
What happens to the bond angles during the change from\( AlCl _{3} to Al_{ 2}Cl _{6}\)?
A Some decrease, some remain the same.
B Some increase, some remain the same.
C They all decrease.
D They all increase.
Answer/Explanation
Ans:C
Question
Valence shell electron pair repulsion theory should be used to answer this question.
Which species are trigonal planar?
1 \(BH_3\)
2 \(CH_3^+\)
3 \(PH_3\)
The responses A to D should be selected on the basis of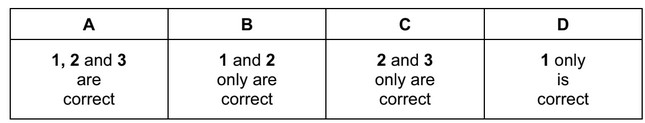
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: B
Question
In which pair do the molecules have the same shape as each other?
- H2O and CO2
- H2O and SCl2
- NH3 and BH3
- SCl2 and BeCl2
Answer/Explanation
Ans:
B
Question
Sodium borohydride, NaBH4, and boron trifluoride, BF3, are compounds of boron.
What are the shapes around boron in the borohydride ion and in boron trifluoride?
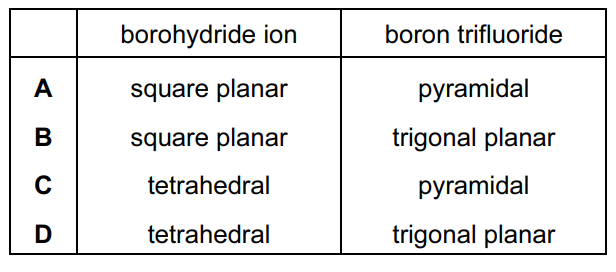
Answer/Explanation
Ans:
D
Question
Two conversions are outlined below.
NH4+ → NH3
C2H4 → C2H6
What similar feature do these two conversions have?
- a lone pair of electrons in the product
- change in oxidation state of an element
- decrease in bond angle of the species involved
- disappearance of a π bond
Answer/Explanation
Ans:
C