Question
(a) [MnCl 4]2- is a complex ion.
(i) Deduce the oxidation state of manganese in [MnCl 4]2-.
(ii) The [MnCl 4]2- complex does not contain any 180° bond angles.
Draw a three-dimensional diagram to show the shape of the [MnCl 4]2- complex. State one bond angle on your diagram.
(b) A solution of cobalt(II) sulfate contains the complex ion [Co(H2O)6]2+.
A solution containing [Co(H2O)6]2+ is reacted separately with an excess of each of NaOH(aq), NH3(aq) and NaCl(aq).
Write an equation for each of these reactions. State one observation that can be made immediately after the reaction, include the colour and state of the cobalt-containing product.
(i) [Co(H2O)6]2+ and an excess of NaOH(aq)
(ii) [Co(H2O)6]2+ and an excess of NH3(aq)
(iii) [Co(H2O)6]2+ and an excess of NaCl(aq)
(iv) Name the type of reaction that occurs in (b)(iii).
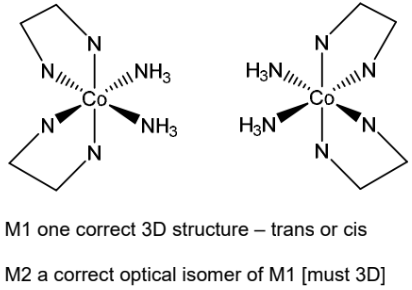
(c) Cobalt forms the complex ion [Co(NH3)2(en)2]2+. The abbreviation en is used for the bidentate ligand 1,2-diaminoethane, H2NCH2CH2NH2. The complex ion shows both geometrical and optical isomerism.
(i) Define the term bidentate ligand.
(ii) Draw three-dimensional diagrams for the two optical isomers of [Co(NH3)2(en)2]2+.
Each en ligand can be represented using 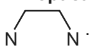
Answer/Explanation
Answer (a)(i) +2 (a)(ii)
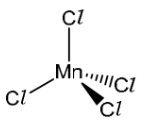
bond angle labelled 109.5°.
(b)(i) [Co(H2O)6]2+ + 2OH– → Co(H2O)4(OH)2 + 2H2O OR [Co(H2O)6]2+ + 2OH– → Co(OH)2 + 6H2O blue precipitate
(b)(ii) [Co(H2O)6]2+ + 6NH3 → [Co(NH3)6]2+ + 6H2O
yellow / brown / straw solution
(b)(iii) [Co(H2O)6]2+ + 4Cl – → [CoCl4]2– + 6H2O
blue solution
(b)(iv) ligand exchange
(c)(i) (a species) that donates two lone pairs to form two dative bonds
to a (transition) metal atom / metal ion
(c)(ii)
Question
Iodates are compounds that contain the IO3– anion.
(a) The IO3– anion is shown.
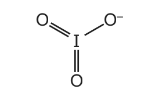
Explain, with reference to the qualitative model of electron-pair repulsion, why the IO3– anion has a pyramidal shape.
(b) The reaction of iodine and hot aqueous sodium hydroxide is similar to that of chlorine and hot aqueous sodium hydroxide. Sodium iodate, NaIO3, is formed as one of the products.
Suggest an equation for the reaction of iodine and hot aqueous sodium hydroxide.
(c) The decomposition of hydrogen peroxide, H2O2, is catalysed by acidified IO3–. H2O2 reduces acidified IO3– as shown.
\(5H_{2}O_{2} + 2H^{+} + 2IO_{3}^{-} \rightarrow I_{2} + 5O_{2} + 6H_{2}O\)
This reaction is followed by the oxidation of I2 by H2O2.
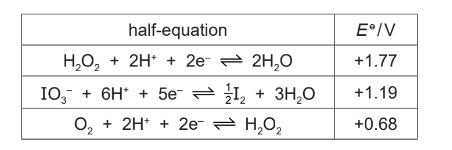
(i) Use the data to show that the separate reactions of H2O2 with IO3– and with I2 are both feasible under standard conditions.
In your answer, give the equation for the reaction of H2O2 with I2.
(ii) Write the overall equation for the decomposition of H2O2 catalysed by acidified IO3– .
(d) A student collects some data for the reaction of H2O2 with acidified IO3– , as shown in the table.
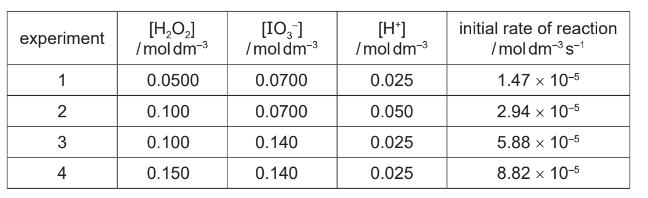
(i) Use the data to determine the order of reaction with respect to [H2O2], [IO3–] and [H+].
Show your reasoning.
(ii) Use your answer to (d)(i) to write the rate equation for this reaction.
(iii) Calculate the value of the rate constant, k, using data from experiment 4 and your answer
to (d)(ii).
Give the units of k.
(e) Pb(IO3)2 is only sparingly soluble in water at 25°C.
The solubility product, Ksp, of Pb(IO3)2 is 3.69 × 10–13mol³ dm-9 at 25°C.
(i) Write an expression for the solubility product of Pb(IO3)2.
(ii) Calculate the solubility, in moldm-3, of Pb(IO3)2 at 25°C.
(f) NH4IO3 is an unstable compound that readily decomposes when warmed. The decomposition
reaction is shown.
\(NH_{4}IO_{3}(s) \rightarrow \frac{1}{2}N_{2} (g)+ \frac{1}{2}N_{2}(g) +\frac{1}{2}I_{2}(g) + 2H_{2}O(l)\) ΔH = -154.6kJ mol-1
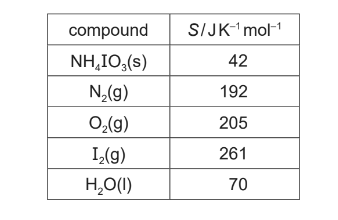
(i) Use the data in the table to calculate the entropy change of reaction,∆S, of the decomposition of NH4IO3(s).
(ii) This reaction is feasible at all temperatures.
Explain why, using the data in (f) and your answer to (f)(i).
Answer/Explanation
Answer: (a) 3 bonding-pair centres and one lone pair (on iodine)
(b) 3I2 + 6NaOH → NaIO3 + 5NaI + 3H2O
(c)(i) M1: Eθcell for IO3– / H2O2 = –0.68 + 1.19 = +0.51 ( ∴ feasible)
M2: Eθcell for H2O2 / I2 = +1.77 – 1.19 = +0.58 ( ∴ feasible)
M3: 5H2O2 + I2 → 4H2O + 2IO3– + 2H+
(c)(ii) 2H2O2 → 2H2O + O2
(d)(i) M1: first order w.r.t. H2O2 AND change in conc. × 1.5 gives increase rate × 1.5 (expts 3 / 4)
M2: first order w.r.t. IO3– AND change in conc. × 2 gives increase rate × 2 (as reaction first order w.r.t. H2O2) (expts 1 / 3)
M3: zeroth order w.r.t. H+ AND change in conc. has no effect on rate (expts 1 / 3 / 4 and 2)
(d)(ii) rate = k[H2O2][IO3– ] ecf
(d)(iii) M1:
k = 8.82 × 10-5 ÷ (0.150 × 0.140) = 4.20 × 10-3 min 2sf ecf
M2: mol-1 dm³ s-1 ecf
(e)(i) Ksp = [Pb 2+][IO3– ]²
(e)(ii) M1: 3.69 × 10-13 = x(2x)² OR x = ∛(3.69 × 10-13 ÷ 4)
M2: = 4.5(2) × 10-5 (mol dm-3) min 2sf ecf
(f)(i) M1: ΔS = 1⁄2(192) + 1⁄2(205) + 1⁄2(261) + 2(70) – 42
M2: (+)427 (J K-1mol-1) ecf
(f)(ii) ΔG (always) negative because
• ΔH < 0 / negative OR exothermic AND
• ΔS > 0 / positive OR – TΔ S < 0 for all T