Question
(a) An aldehyde, an alkane and a carboxylic acid, all of similar volatility, are mixed together. The mixture is then analysed in a gas chromatograph.
The gas chromatogram produced is shown.
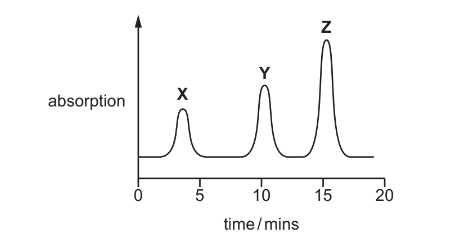
The separation of the compounds depends on their relative solubilities in the stationary phase.
The stationary phase is a liquid alcohol.
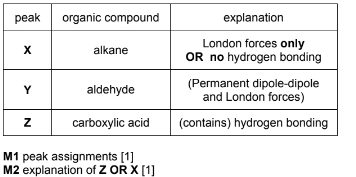
(i) Complete the table to suggest which compound in the mixture is responsible for each peak X, Y and Z. Explain your answer by reference to the intermolecular forces of the compounds.
(ii) A student calculates the areas underneath the three peaks in the chromatogram.
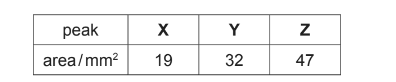
The area underneath each peak is proportional to the mass of the respective compound.
Calculate the percentage by mass in the original mixture of the compound responsible for peak Z.
(b) (i) The mass spectrum of a halogenoalkane containing one chlorine atom or bromine atom will show an additional peak at M+2.
State the isotopes of chlorine and bromine responsible for M+2 peaks.
(ii) The mass spectrum of bromochloromethane, CH2BrCl, has a molecular ion peak, M, at an m/e value of 128. It also has M+2 and M+4 peaks.
Suggest the identity of the molecular ions that give rise to these peaks.
(c) Halogenoalkanes can be formed from the reaction of an alkene with a hydrogen halide. Methylpropene reacts with hydrogen bromide to form 2-bromo-2-methylpropane.
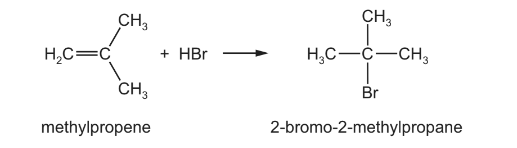
(i) Draw the mechanism of this reaction. Include all relevant curly arrows, dipoles and charges.
(ii) 1-bromo-2-methylpropane is also formed in this reaction.
Explain why 2-bromo-2-methylpropane will be the major product in this reaction.
(d) (i) Explain what is meant by the term partition coefficient, Kpartition.
(ii) The partition coefficient of organic compound H between dichloromethane and water is 4.75.
● 2.50g of compound H was dissolved in water and made up to 100cm3 in a volumetric flask.
● 50cm3 of this aqueous solution were shaken with 10cm3 of dichloromethane.
Calculate the mass of compound H that was extracted into the dichloromethane.
Answer/Explanation
Answer: (a)(i)
(a)(ii) % of Z = 47/98 = 48%
(b)(i) 37Cl and 81Br
(b)(ii) M peak CH235Cl79Br
M+2 peak CH237Cl79Br OR CH235Cl81Br M+4 peak CH237Cl81Br
two correct scores 1 mark
all 3 correct scores 2 marks
(c)(i)
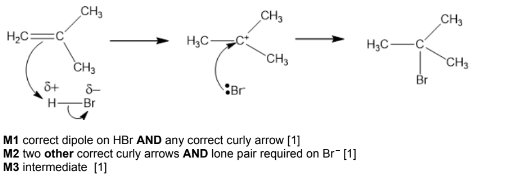
(c)(ii) (major product is) formed via the most stable tertiary carbocation / intermediate
OR tertiary halogenoalkane formed via more stable carbocation / intermediate
(d)(i) M1 ratio of the concentrations of solute in two (immiscible) solvents [1]
M2 at equilibrium [1]
(d)(ii) Kpartition = (x/10)/(1.25-x/50) [1]
4.75(1.25-x) = 5x
x = 5.9375/9.75 = 0.61 g [1]
Question
The following compounds were all found to be components of a sample of petrol.
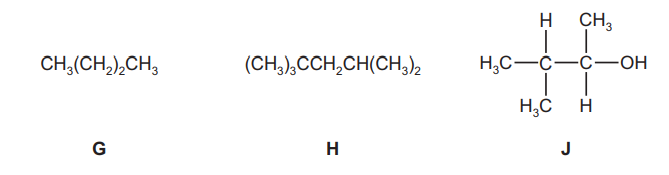
(a) (i) Give the molecular formula of compound G.
……………………………………………………………………………………………………………………… [1]
(ii) Give the empirical formula of compound H.
……………………………………………………………………………………………………………………… [1]
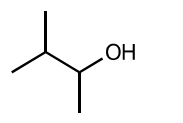
(iii) Draw the skeletal formula of compound J.[1]
(b) Write an equation to represent the complete combustion of compound H.
……………………………………………………………………………………………………………………………. [1]
(c) Fossil fuels are often contaminated with sulfur.
State and explain why supplies of fossil fuels that contain sulfur pose a problem to the environment.
(d) The boiling points of compounds G, H and J are shown below.
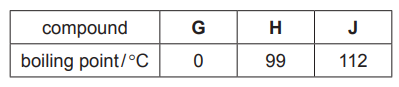
Explain the differences in the boiling points of the three compounds.
(e) Compound $\mathbf{J}$ can be produced from 2-chloro-3-methylbutane, $\mathrm{C}_5 \mathrm{H}_{11} \mathrm{Cl}$.
Give the reagent(s) and conditions for this reaction.[1] [Total: 11]
▶️Answer/Explanation
Ans:
(a) (i) $\underline{\mathrm{C}}_4 \underline{H}_{10}$
(ii) $\quad \underline{\mathrm{C}}_4 \mathrm{H}_9$
(iii)
(b) $\quad \mathrm{C}_8 \mathrm{H}_{18}+12 \frac{1}{2} \mathrm{O}_2 \rightarrow 8 \mathrm{CO}_2+9 \mathrm{H}_2 \mathrm{O}$
(c) sulfur dioxide would be produced on combustion
(which contributes to) acid rain
(d) M1 = H has more/ greater/ stronger van der Waals’/ intermolecular forces than G / ora
M2 = (because)
H has more electrons (thanG)
M3= J has hydrogen bonding (between molecules)
M4 = strong(er)/ great(er) forces require AND high/ more energy to overcome
(e) NaOH(aq)