Question
Which dot-and-cross diagram is correct for \(Al_2Cl_6\)?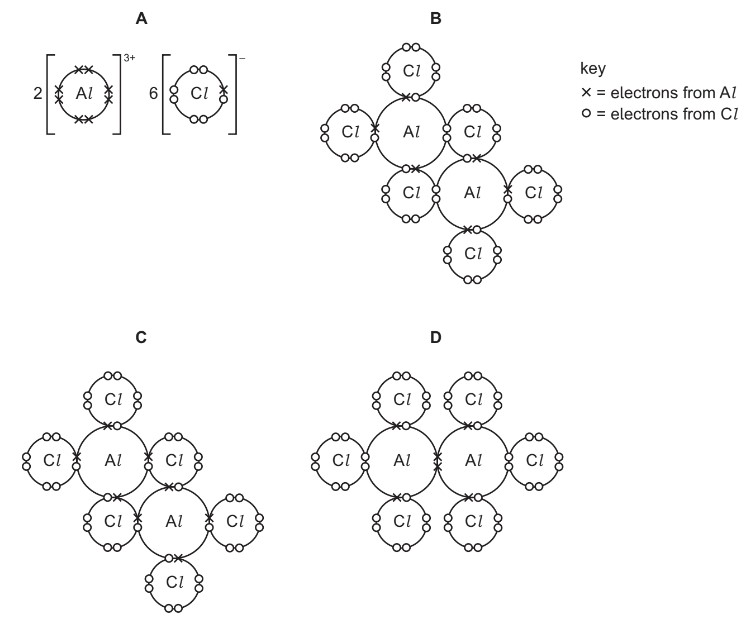
Answer/Explanation
Ans: B
Question
In which structure are three atoms bonded together in a straight line?
A poly(ethene), \(-(-CH_2CH_2-)_n-\)
B propane, \(C_3H_8\)
C silicon tetrachloride, \(SiCl_4\)
D sulfur hexafluoride, \(SF_6\)
Answer/Explanation
Ans: D
Question
Which molecules contain at least one unpaired electron?
1 NO
2 NO2
3 NH3
Answer/Explanation
Answer B
Question
The eight species that follow all have covalent bonds.
In which pair do the species have different shapes from each other?
A BeCl2 and CO2
B CH4 and NH4+
C NH3 and BF3
D SCl2 and H2O
Answer/Explanation
Answer:
C
Question
In which species is there a lone pair of electrons?
A CH3 B CH3+ C CH3– D CH4
Answer/Explanation
Answer C
Question
Which molecule contains six bonding electrons?
A \(C_2H_4\) B \(H_2S\) C \(NCl_3\) D \(SF_6\)
Answer/Explanation
Ans: C
Question
Pollutant oxide Y, which contains non-metallic element X, is formed in a car engine. Further oxidation of Y to Z occurs in the atmosphere. In this further oxidation, 1mol of Y reacts with 0.5mol of gaseous oxygen molecules. X could be either nitrogen or sulfur. Which statements about X, Y and Z can be correct?
1 The oxidation number of X increases by two from Y to Z.
2 Y has an unpaired electron in its molecule.
3 Y is a polar molecule.
The responses A to D should be selected on the basis of

Answer/Explanation
Ans:A
Question
Use of the Data Booklet is relevant to this question.
Free-radicals play an important part in reactions involving the destruction of the ozone layer and the substitution of alkanes by chlorine.
Some free-radicals contain two unpaired electrons. Such species are called diradicals. Which species are diradicals?
1 O
2 Cl
3 \(CH_3\)
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: D
Question
In which species does the underlined atom have an incomplete outer shell?
A BF3 B CH3– C F2O D H3O+
Answer/Explanation
Ans:
A