Question
When ClNO2 reacts with NO an equilibrium is established.
\(ClNO_{2}(g) + NO(g) \rightleftharpoons NO_{2}(g) + ClNO(g)\)
In each ClNO2 molecule the nitrogen atom is bonded to the chlorine atom and bonded to each of
the oxygen atoms separately.
(a) Draw a ‘dot-and-cross’ diagram for the ClNO2 molecule.
(b) The reaction between ClNO2 and NO is first order with respect to each reactant.
(i) Write the rate equation for this reaction.
(ii) Deduce the units of the rate constant, k, when the concentrations of both gases are
measured in moldm-3 and the rate is measured in moldm-3 s-1
(iii) State and explain whether or not the reaction could take place in a single step.
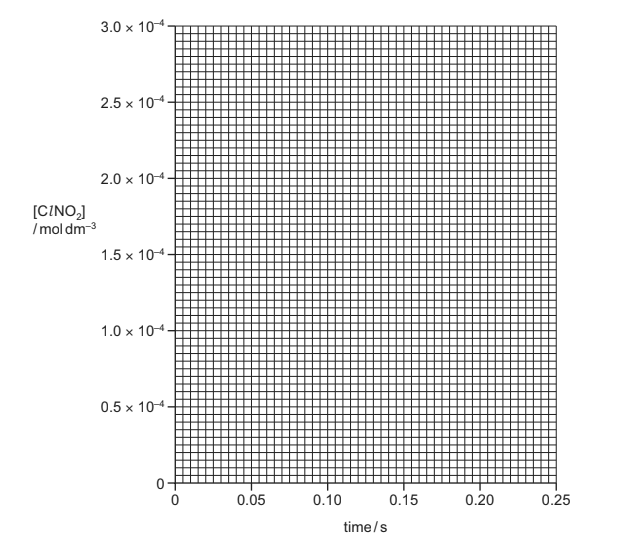
(c) An experiment is carried out in which the initial [ClNO2] is 2.0 × 10-4moldm-3. A large excess
of NO is used. The initial rate of reaction is 1.0 × 10-4moldm-3 s-1. The rate of the reaction is
assumed to be constant for the first 0.20 seconds.
(i) Draw a graph on the grid to show how the concentration of ClNO2 varies for the first
0.20 seconds.
(ii) Deduce the concentration of the NO2 product at 0.20 seconds.
(iii) After 20 seconds the concentration of ClNO2 remains constant.
Explain this observation.
Answer/Explanation
Answer: (a)

(b)(i) (rate =) k[ClNO2][NO]
(b)(ii) mol-1 dm3 s-1
(b)(iii) Yes AND number of moles of reactants in overall equation is the same as order in rate equation
(c)(i) • straight line with a negative gradient • starting at 2.0 × 10-4 • reaches at 1.8 × 10-4 at 0.2 seconds
Award 1 mark for two points, award 2 marks for all three points
(c)(ii) 2 × 10-5 (mol dm-3)
(c)(iii) The reaction has reached equilibrium
Question
(a) Hydrogen cyanide, HCN, is a weak acid in aqueous solution.
HCN(aq) \(\rightleftharpoons \) H+(aq) + CN–(aq) Ka = 6.2 × 10–10moldm–3
(i) Calculate the pH of 0.10moldm–3 HCN(aq).
pH = ………………………… [2]
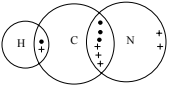
(ii) Draw a ‘dot-and-cross’ diagram to represent the bonding in the hydrogen cyanide molecule.
Show the outer shell electrons only.
[1]
(iii) State the hybridisation of the carbon and nitrogen atoms in hydrogen cyanide, and give
the H–C–N bond angle.
hybridisation of C ………………………………………………………………………………………………….
hybridisation of N ………………………………………………………………………………………………….
H–C–N bond angle ……………………………………………………………………………………………….
[2]
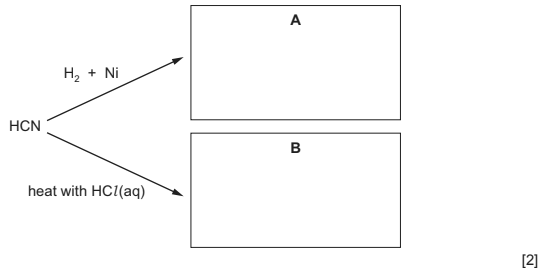
(iv) Suggest structures for the organic products A and B in the following reactions. Assume
that HCN reacts in a similar way to RCN.
(b) Adding a measured quantity of KCN to a solution of NiCl2 produces the complex [Ni(CN)2Cl2]x.
(i) Deduce the overall charge, x, on this complex.
x = ………………………… [1]
The complex can exist as two separate isomers with the same geometry (shape) around the
nickel ion.
(ii) State the type of isomerism shown by these isomers.
……………………………………………………………………………………………………………………… [1]
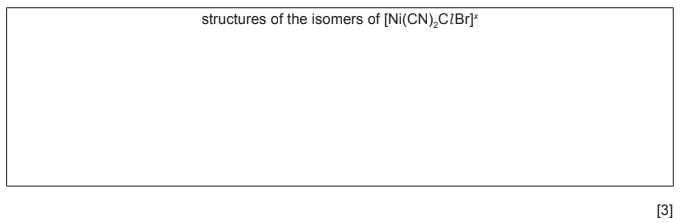
(iii) If bromide ions are present in the solution, the complex [Ni(CN)2ClBr]x can form.
Assuming that [Ni(CN)2ClBr]x has the same geometry as [Ni(CN)2Cl2]x, state the number
of isomers of [Ni(CN)2ClBr]x that could exist, and draw their structures in the box.
● number of isomers of [Ni(CN)2ClBr]x …………………………
(c) An aqueous solution of KCN is gradually added to a solution of NiSO4 until the KCN is in
excess. The following series of reactions takes place.

● The oxidation state of nickel does not change during these reactions.
● None of C, D or E contains sulfur.
● C contains no potassium.
● The K:Ni ratio in D is 2:1.
● The K:Ni ratio in E is 3:1.
Use the information to suggest the formulae of C, D and E.
C ………………………………………………………………………………………………………………………………
D ………………………………………………………………………………………………………………………………
E ………………………………………………………………………………………………………………………………
[3]
[Total: 15]
▶️Answer/Explanation
Answer:
(a)(i) [H+] =√(Ka. c) =√(6.2× 10–10× 0.1)
[H+] = 7.9× 10–6
pH = –log[H+] = 5.1(0)
(a)(ii)
(a)(iii) C: sp and N: sp
angle 180°
(a)(iv) A is CH3NH2
B is HCO2H
(b)(i) 2-
(b)(ii) geometrical / cis-trans
(b)(iii) 2 isomers
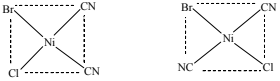
(must be clearly square planar)
(c) C is Ni(CN)2
D is K2Ni(CN)4
E is K3Ni(CN)5