Question
(a) Compare the relative acidities of benzoic acid \((C_6H_5COOH)\), phenylmethanol \((C_6H_5CH_2OH)\), and phenol \((C_6H_5OH)\).
Explain your reasoning.
……………………………………. > ……………………………………. > …………………………………….
most acidic least acidic
(b) A series of nine separate experiments is carried out as shown in Table 5.1.
Complete the table by placing a tick (✓) in the relevant box if a reaction occurs. Place a cross (✗) in the box if no reaction occurs.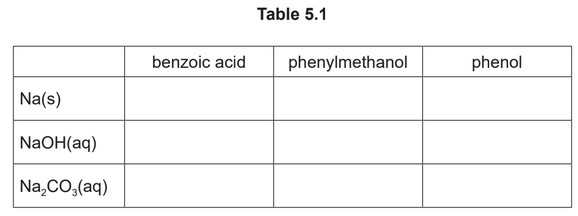
(c) (i) Benzoyl chloride, \(C_6H_5COCl\), can be synthesised by the reaction of benzoic acid with either \(PCl_5\) or \(SOCl_2\).
Complete the equations for these reactions.
reaction 1 \(C_6H_5COOH + PCl_5 → C_6H_5COCl\) + …………………… + ……………………
reaction 2 \(C_6H_5COOH + SOCl_2 → C_6H_5COCl\) + …………………… + ……………………
(ii) Use your answer to (c)(i) to suggest why it is easier to isolate, in a pure form, the \(C_6H_5COCl\) from reaction 2 compared to reaction 1.
(d) Benzoyl chloride is hydrolysed by water at room temperature to form benzoic acid.
(i) Complete the diagram to show the mechanism for the reaction between \(C_6H_5COCl\) and \(H_2O\).
Include charges, dipoles, lone pairs of electrons and curly arrows as appropriate.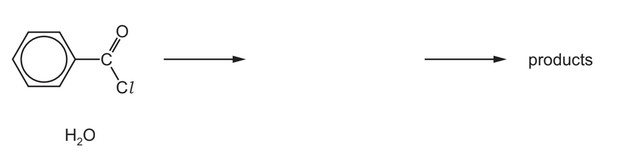
(ii) Name the type of mechanism you showed in (d)(i).
(e) Acyl chlorides react with sodium carboxylates to form acid anhydrides as shown in Fig. 5.1.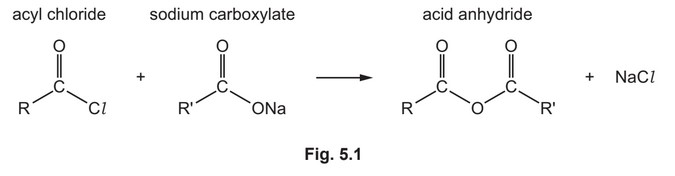
The condensation polymers, polyanhydride and polyester, are formed by similar methods.
The repeat unit for a polyanhydride is shown in Fig. 5.2.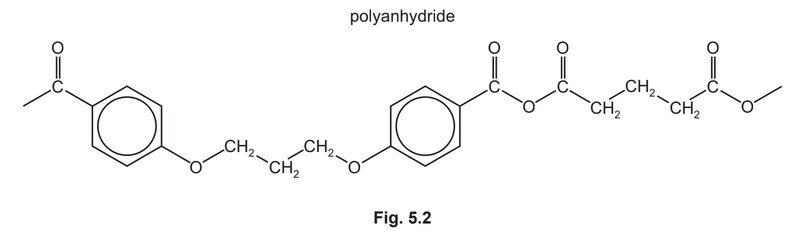
(i) Use Fig. 5.1 and Fig. 5.2 to suggest the structures of the two monomers used to make this polyanhydride.
(ii) Polyanhydrides are biodegradable polymers.
Suggest how this polyanhydride can be degraded.
Answer/Explanation
Answer:
(a) M1 benzoic acid > phenol > phenylmethanol
M2 / M3 Any two of:
in benzoic acid negative inductive effect of C=O AND O-H bond is weakened
OR due to delocalisation of minus charge by C=O / 2O carboxylate ion is stabilised
in phenol lone pair on oxygen is delocalised into the ring AND O-H bond is weakened
in phenyl methanol positive inductive effect of \(CH_2\) group AND O-H bond is strengthened
(b) 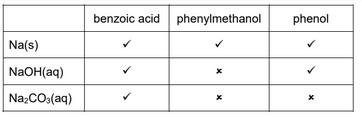
Three correct for one mark, six correct for two marks, nine correct for three marks
(c) (i) \(POCl_3\) and HCl AND \(SO_2\) and HCl
(ii) all the by-products / SO2 and HCl are gaseous OR no liquid by-products formed
(d) (i) 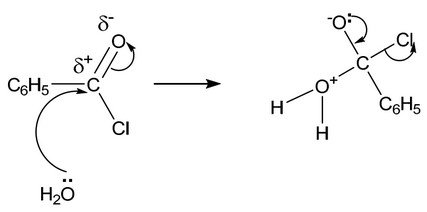
On the left-hand side:
lone pair on O
correct arrow from O to C (of C=O)
dipole on C=O
correct arrow on C=O
M1 / M2 Two correct for one mark, four correct for two marks
On the right-hand side:
M3 correct intermediate
M4 arrow from lone pair on O– to C-O bond AND arrow from C-Cl to Cl
(ii) addition-elimination
(e) (i) 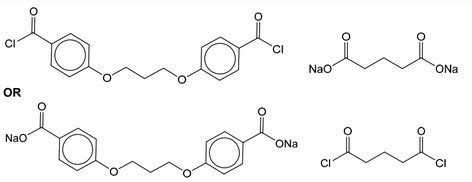
(ii) hydrolysis OR heating in dilute acid / alkali
Question
The three substances shown all have some acidic properties.
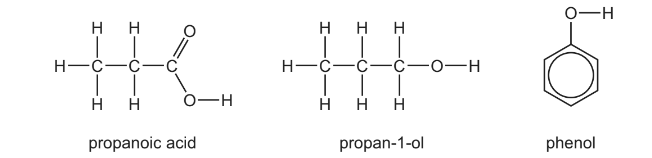
(a) Write an equation for the reaction between propan-1-ol and sodium metal.
(b) (i) Give the order of the relative acidities of propanoic acid, propan-1-ol and phenol, stating the most acidic first.
(ii) Explain your answer to (i).
(c) Methanoic acid, HCO2H, has a similar acid strength to propanoic acid.
Describe a chemical test to distinguish between these two acids. Name the acid which gives a positive result in this test and describe the observations that would be made.
(d) The ester phenyl propanoate, C2H5CO2C6H5, can be made from phenol and propanoic acid in a two-step synthesis. The first step produces an acyl chloride.
For this two-step synthesis,
● draw the structure of the product of the first step,
● state the reagents and conditions needed for each step of the synthesis.
(e) An unknown compound, Z, is propan-1-ol, propanal or propanoic acid. The proton NMR spectrum of Z dissolved in CDCl3 is shown.
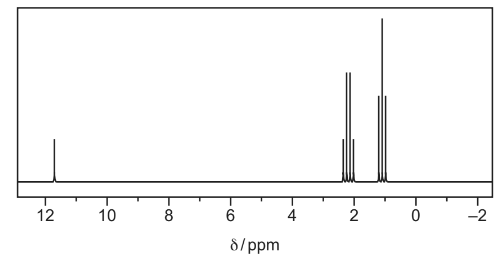
(i) From the proton NMR spectrum, identify Z.
(ii) State one feature that would be seen, and why, in the proton NMR spectra of each of the two compounds that are not Z.
Answer/Explanation
Answer: (a) 2C3H7OH + 2Na → 2C3H7ONa + H2
(b)(i) propanoic acid, phenol, propan-1-ol
(b)(ii) • propan-1-ol: O-H bond strengthened by positive inductive effect of alkyl group OR propoxide ion is destabilised by positive inductive effect of alkyl group
• phenol: O-H bond weakened by negative inductive effect of ring OR phenoxide ion is stabilised by delocalisation of
oxygen lone pair into ring
• propanoic acid: O-H bond weakened by negative inductive effect of C=O OR propanoate ion is stabilised by
delocalisation of minus charge by C=O
1 mark for a correct explanation, max 2 marks
(c) Tollens’ reagent or Fehling’s reagent
methanoic acid gives a silver mirror/solid with Tollen’s reagent
OR red / orange ppt / solid with Fehlings’ reagent
(d) PCl5 or PCl3 (+heat) or SOCl2 (added to propanoic acid)
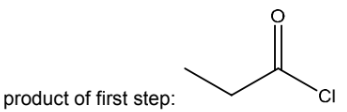
add product of first step to phenol in NaOH
(e)(i) propanoic acid
(e)(ii) propan-1-ol would have peak at 0.5–6.0 because of OH group
propanal would have peak at 9.3–10.5 because of CHO / aldehyde