Question
Phenol, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH}$, is a weak acid.
(a) Phenol can be made from phenylamine, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2$.
Give the reagents and conditions for this reaction. [5]
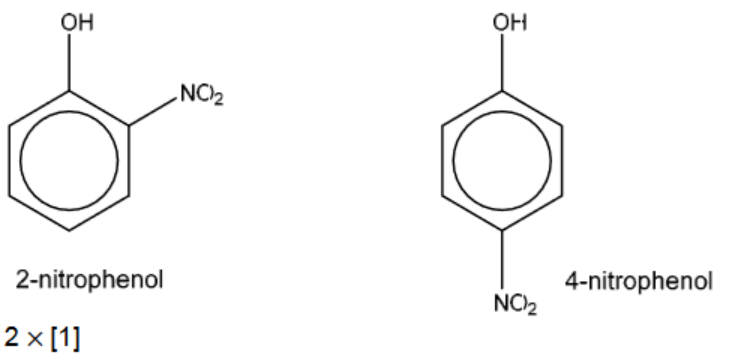
(b) Phenol reacts with dilute aqueous nitric acid under room conditions to give a mixture of two isomeric products with molecular formula $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_3$.
Use the Data Booklet to draw the structural formulae of these two products in the boxes and name each product.
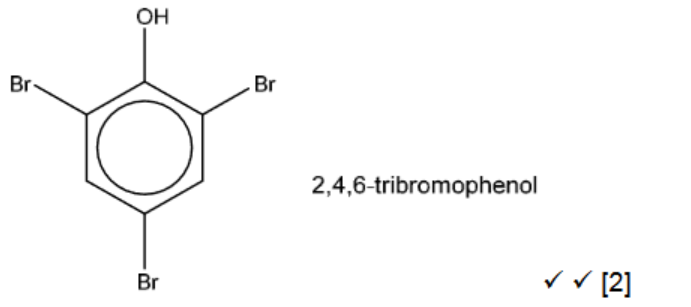
(c) Phenol reacts with an excess of aqueous bromine.
(i) Draw and name the organic product of this reaction in the box
(ii) Describe two visual observations that can be made when phenol reacts with an excess of aqueous bromine.
observation 1…………………………………………..
observation 2…………………………………………………..[1]
(d) Write an equation for a neutralisation reaction in which phenol behaves as an acid..[1]
(e) Water, phenol and ethanol can all behave as acids.
Place these three compounds in order of acidity, starting with the most acidic. Explain your answer.
…………………………….. > ……………………………… > ………………………………
most acidic least acidic [3] [Total: 11]
▶️Answer/Explanation
Ans:
(a) M1: $\mathrm{HNO}_2$ OR NaNO${ }_2+\mathrm{HCl} l$ [1]
M2: $\mathrm{T} \geqslant 10^{\circ} \mathrm{C} /$ warm AND water [1]
(b)
(c)(i)
7(c)(ii) bromine is decolourised AND white precipitate is formed
BOTH [1]
(d)
$
\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH}+\mathrm{NaOH} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{ONa}+\mathrm{H}_2 \mathrm{O} \text { [1] }
$
ALLOW any equation for phenol acting as an acid
(e)
phenol>water>ethanol [1]
• (phenol:) lone pair on oxygen is delocalised into the benzene ring
• (ethanol:) positive inductive effect / electron donating effect of alkyl / ethyl group
• correct statement about stabilisation of anion/ conjugate base OR weakening of O-H bonds once in the context of
phenol / ethanol
• correct statement about ease of proton/$H ^{+}$ donation in the context of phenol / ethanol [2]
Two correct statements = 1 mark
Question
(a) Compare the relative acidities of benzoic acid \((C_6H_5COOH)\), phenylmethanol \((C_6H_5CH_2OH)\), and phenol \((C_6H_5OH)\).
Explain your reasoning.
……………………………………. > ……………………………………. > …………………………………….
most acidic least acidic
(b) A series of nine separate experiments is carried out as shown in Table 5.1.
Complete the table by placing a tick (✓) in the relevant box if a reaction occurs. Place a cross (✗) in the box if no reaction occurs.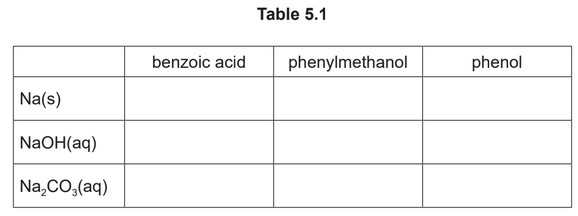
(c) (i) Benzoyl chloride, \(C_6H_5COCl\), can be synthesised by the reaction of benzoic acid with either \(PCl_5\) or \(SOCl_2\).
Complete the equations for these reactions.
reaction 1 \(C_6H_5COOH + PCl_5 → C_6H_5COCl\) + …………………… + ……………………
reaction 2 \(C_6H_5COOH + SOCl_2 → C_6H_5COCl\) + …………………… + ……………………
(ii) Use your answer to (c)(i) to suggest why it is easier to isolate, in a pure form, the \(C_6H_5COCl\) from reaction 2 compared to reaction 1.
(d) Benzoyl chloride is hydrolysed by water at room temperature to form benzoic acid.
(i) Complete the diagram to show the mechanism for the reaction between \(C_6H_5COCl\) and \(H_2O\).
Include charges, dipoles, lone pairs of electrons and curly arrows as appropriate.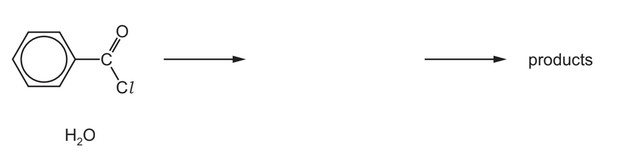
(ii) Name the type of mechanism you showed in (d)(i).
(e) Acyl chlorides react with sodium carboxylates to form acid anhydrides as shown in Fig. 5.1.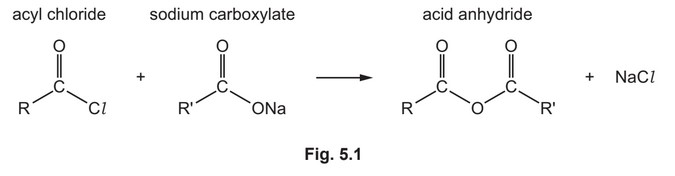
The condensation polymers, polyanhydride and polyester, are formed by similar methods.
The repeat unit for a polyanhydride is shown in Fig. 5.2.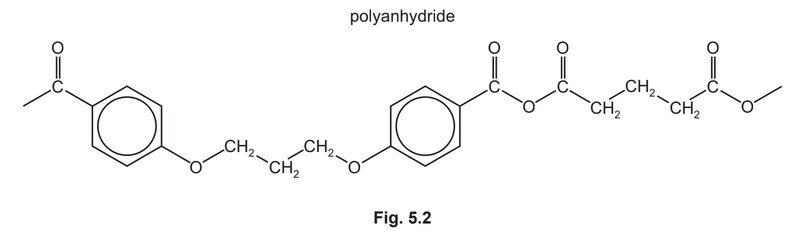
(i) Use Fig. 5.1 and Fig. 5.2 to suggest the structures of the two monomers used to make this polyanhydride.
(ii) Polyanhydrides are biodegradable polymers.
Suggest how this polyanhydride can be degraded.
Answer/Explanation
Answer:
(a) M1 benzoic acid > phenol > phenylmethanol
M2 / M3 Any two of:
in benzoic acid negative inductive effect of C=O AND O-H bond is weakened
OR due to delocalisation of minus charge by C=O / 2O carboxylate ion is stabilised
in phenol lone pair on oxygen is delocalised into the ring AND O-H bond is weakened
in phenyl methanol positive inductive effect of \(CH_2\) group AND O-H bond is strengthened
(b) 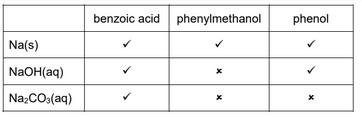
Three correct for one mark, six correct for two marks, nine correct for three marks
(c) (i) \(POCl_3\) and HCl AND \(SO_2\) and HCl
(ii) all the by-products / SO2 and HCl are gaseous OR no liquid by-products formed
(d) (i) 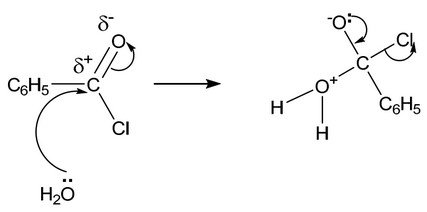
On the left-hand side:
lone pair on O
correct arrow from O to C (of C=O)
dipole on C=O
correct arrow on C=O
M1 / M2 Two correct for one mark, four correct for two marks
On the right-hand side:
M3 correct intermediate
M4 arrow from lone pair on O– to C-O bond AND arrow from C-Cl to Cl
(ii) addition-elimination
(e) (i) 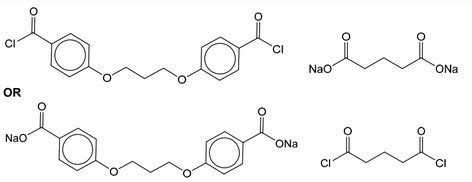
(ii) hydrolysis OR heating in dilute acid / alkali