Question
(a) The mass spectrum of compound X, C5H10O2, is recorded.
The peak heights of the M and M+1 peaks are 22.65 and 1.25 respectively.
(i) Use these data to show that there are five carbon atoms present in one molecule of X.
Show your working.
(ii) The mass spectrum has a peak at m/e = 57.
Complete the equation to show the fragmentation of X to produce this peak.
[C5H10O2] + ___________________ + _____________________
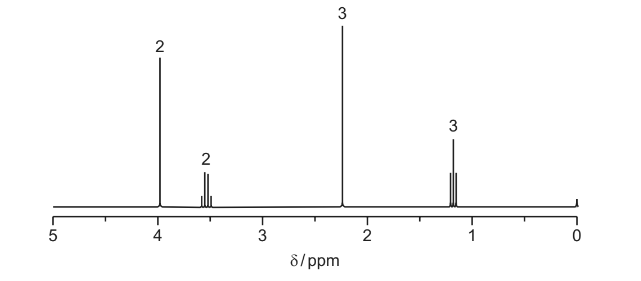
(i) By considering both the relative peak areas and their δ values, use the Data Booklet to
● deduce the part of the molecule that produces the peak at δ2.2,
● deduce the part of the molecule that produces the peaks at δ1.2 and δ3.5,
● deduce the part of the molecule that produces the peak at δ4.0.
(ii) When reacted with aqueous alkaline iodine, X produces a yellow precipitate.
Use this information and your answers to (c)(i) to suggest a structure for X.
(d) Compound W is an ester with the molecular formula C5H10O2.
The proton NMR spectrum of W contains only two peaks.
The relative areas of these two peaks are in the ratio 9:1.
Suggest a structure for this ester, W.
(e) Compound V is a carboxylic acid which contains a chiral centre. It also has the molecular
formula C5H10O2.
(i) Explain what is meant by the term chiral centre.
(ii) Suggest a structure for V.
Answer/Explanation
Answer: (a)(i) no. of carbons = 100 × 1.25 / (22.65 × 1.1) (= 5.02)
(a)(ii) M1: C2H5O
M2: C3H5O+ (positive sign required for m / e = 57 fragment)
(b) TMS: Reference
CDCl3: Solvent
(c)(i) M1: CH3CO
M2: CH3CH2O
M3: (CO)CH2O
(c)(ii) CH3COCH2OCH2CH3
(d) HCO2C(CH3)3
(e)(i) this is a (carbon) atom which has four different atoms or groups attached to it
(e)(ii) CH3CH2CH(CH3)COOH
Question
(a) Ethanedioate ions, C2O42–, are bidentate ligands.
Explain what is meant by the term ligand.
(b) Cr3+(aq) and C2O42–(aq) ions form the complex ion [Cr(C2O4)2(H2O)2]–.
Draw two stereoisomers of this complex ion.
You may use ![]() to represent C2O42–.
to represent C2O42–.
(c) The solubility of calcium ethanedioate, CaC2O4, is 6.65 × 10–3 gdm–3 at 298K.
(i) Write an expression for the solubility product, Ksp, of CaC2O4. Include its units.
(ii) Calculate the numerical value of Ksp CaC2O4 at 298K. Give your answer in standard form
to two significant figures.
Answer/Explanation
Answer: (a) species that forms dative bond(s) to a (central) metal atom / ion
(b) 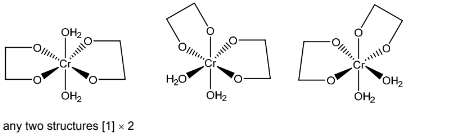
(c)(i) Ksp = [Ca2+][C2O42–] [1]
units mol2 dm–6 [1]
(c)(ii) [Ca2+] = [C2O42–] = 6.65 × 10–3/128.1 = 5.19 × 10–5 mol dm–3 [1] Ksp = (5.19 × 10–5)2 = 2.7 × 10–9 mol2 dm–6 [1]