Question
(a) Ethanedioic acid, $\mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2$, occurs in many vegetables. The amount that occurs in spinach can be estimated as follows.
- $\quad 40.0 \mathrm{~g}$ of spinach leaves are crushed and mixed with distilled water, using a mortar and pestle.
- The mixture is filtered, and the leaves are washed with a little more water.
- The combined filtrate and washings are made up to $100.0 \mathrm{~cm}^3$ with water.
- A $25.0 \mathrm{~cm}^3$ portion of the resulting solution is added to a conical flask, along with an excess of dilute sulfuric acid.
- The acidified solution is warmed, and then titrated with $0.0200 \mathrm{moldm}^{-3} \mathrm{KMnO}_4$.
The equation for the reaction between ethanedioic acid and acidified manganate(VII) ions is shown.
$
2 \mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+5 \mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2 \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}
$
In the titration, $15.20 \mathrm{~cm}^3$ of $\mathrm{KMnO}_4$ was required to reach the end-point.
Calculate the percentage by mass of ethanedioic acid in the spinach leaves.
percentage of ethanedioic acid = ……………………….. % [3]
(b) Ethanedioic acid can be converted into ethanedioyl chloride:
$
\mathrm{HO}_2 \mathrm{CCO}_2 \mathrm{H} \rightarrow \mathrm{C} l \mathrm{OCCOC} l
$
(i) State a suitable reagent for this reaction.[1]
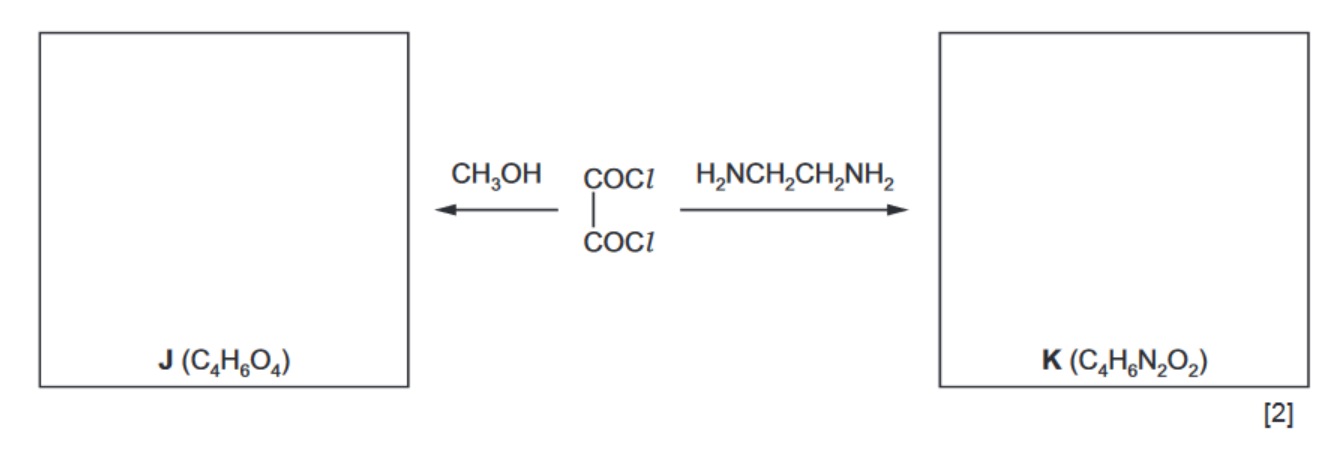
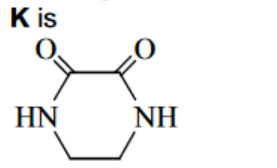
(ii) For the reactions of ethanedioyl chloride below, suggest the structures of compounds $\mathbf{J}$ and $\mathbf{K}$ and draw them in the boxes.
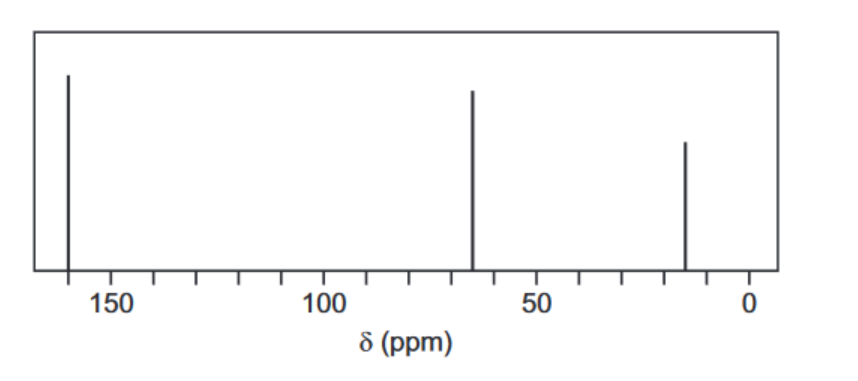
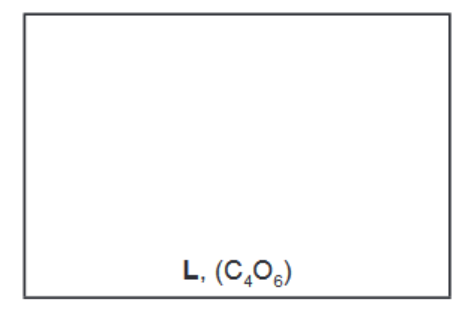
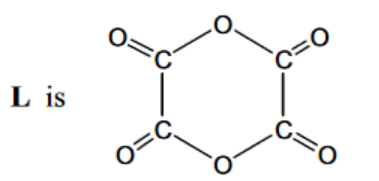
(c) When ethanedioyl chloride is reacted with silver ethanedioate, $\mathrm{AgO}_2 \mathrm{CCO}_2 \mathrm{Ag}$, in ethoxyethane at $-30^{\circ} \mathrm{C}$, an oxide of carbon, $L$, is formed. The molecule of $L$ has no overall dipole and has molecular formula $\mathrm{C}_4 \mathrm{O}_6$.
The carbon-13 NMR spectrum of a solution of $\mathrm{L}$ in ethoxyethane, $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OCH}_2 \mathrm{CH}_3$, is shown below.
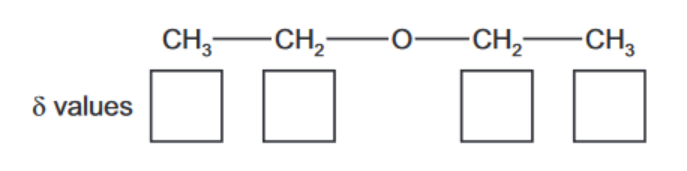
(i) Use the Data Booklet to state in the boxes below the $\delta$ values for the peaks in the spectrum which are due to the carbon atoms in ethoxyethane.
(i) Explain what the rest of the carbon-13 NMR spectrum indicates about the structure of L. ……………………………………………………………………………………………………………………… [1]
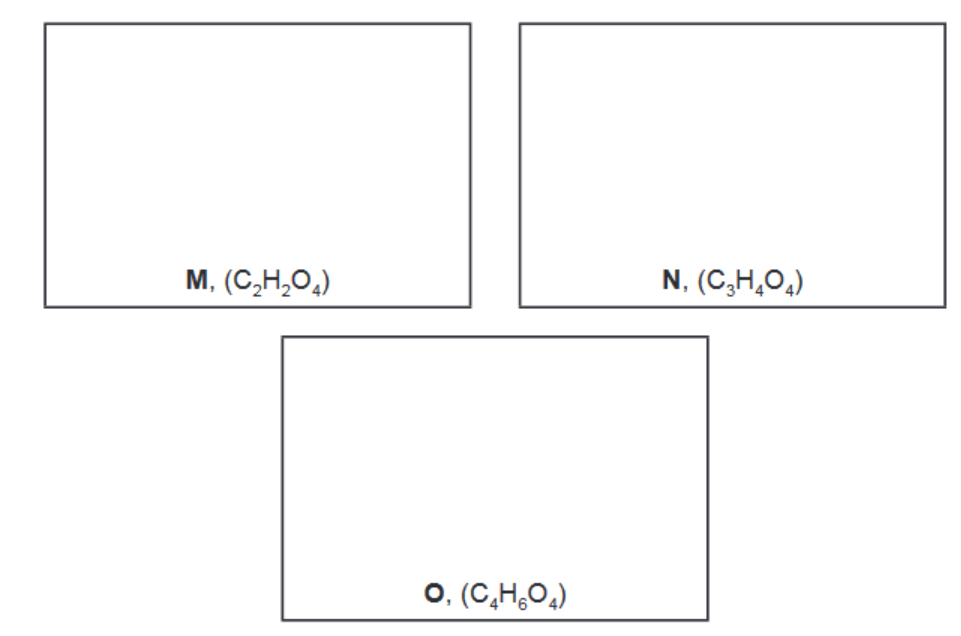
(d) When pure $\mathrm{L}$ is reacted with an excess of $\mathrm{CH}_3 \mathrm{OH}$, a mixture of three compounds is formed.
$\begin{gathered}\mathbf{L} \\ \left(\mathrm{C}_4 \mathrm{O}_6\right)\end{gathered}$ and $\mathrm{CH}_3 \mathrm{OH} \rightarrow \underset{\left(\mathrm{C}_2 \mathrm{H}_2 \mathrm{O}_4\right)}{\rightarrow} \quad \begin{gathered}\mathbf{M} \\ \left(\mathrm{C}_3 \mathrm{H}_4 \mathrm{O}_4\right)\end{gathered}$ and $\begin{gathered}\mathbf{O} \\ \left(\mathrm{C}_4 \mathrm{H}_6 \mathrm{O}_4\right)\end{gathered}$
M is formed as one of the products when either N or O is heated with aqueous acid.
The table gives information of the peaks recorded in the carbon-13 NMR spectra of M, N and O
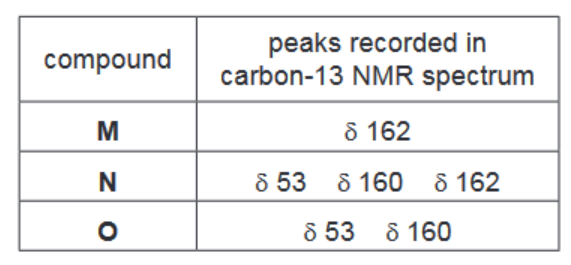
i) Suggest the structures of M, N and O
ii) Suggest a structure for L that fits all the data given in (c) and (d).
 [1] [Total: 13]
[1] [Total: 13]
▶️Answer/Explanation
Ans:
(a)
$
\begin{aligned}
& \mathrm{n}\left(\mathrm{MnO}_4{ }^{-}\right)=0.02 \times 15.2 / 1000=3.04 \times 10^{-4} \mathrm{~mol} \\
& \mathrm{n}\left(\mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2\right)=3.04 \times 10^{-4} \times 5 / 2=7.6 \times 10^{-4}\left(\text { in } 25 \mathrm{~cm}^3\right)=3.04 \times 10^{-3} \mathrm{~mol} \text { in } 100 \mathrm{~cm}^3 \\
& \mathrm{M}_{\mathrm{r}}=24+64+2=90 \\
& \text { mass of } \mathrm{C}_2 \mathrm{O}_4 \mathrm{H}_2=3.04 \times 10^{-3} \times 90 \\
& =0.2736 \mathrm{~g}(0.274) \\
& \text { percentage }=0.2736 \times 100 / 40=0.68 \%
\end{aligned}
$
(b) (i) $\mathrm{SOCl}_2$ or $\mathrm{PCl}_5$ or $\mathrm{PCl}_3$
(ii) $\mathbf{J}$ is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{COOCH}_3$
(c) (i) $\quad \begin{aligned} & \mathrm{CH}_3 \text { at } \delta 15 \\ & \mathrm{CH}_2 \mathrm{O} \text { at } \delta 65\end{aligned}$
(ii) Only one peak, so only one type / environment of C atom
(d) (i) $\mathbf{M}$ is $\mathrm{HO}_2 \mathrm{C}-\mathrm{CO}_2 \mathrm{H}$
$\mathbf{N}$ is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{CO}_2 \mathrm{H}$
O is $\mathrm{CH}_3 \mathrm{OCO}-\mathrm{COOCH}_3$
(ii)
Question
(a) Compare the relative acidities of benzoic acid \((C_6H_5COOH)\), phenylmethanol \((C_6H_5CH_2OH)\), and phenol \((C_6H_5OH)\).
Explain your reasoning.
……………………………………. > ……………………………………. > …………………………………….
most acidic least acidic
(b) A series of nine separate experiments is carried out as shown in Table 5.1.
Complete the table by placing a tick (✓) in the relevant box if a reaction occurs. Place a cross (✗) in the box if no reaction occurs.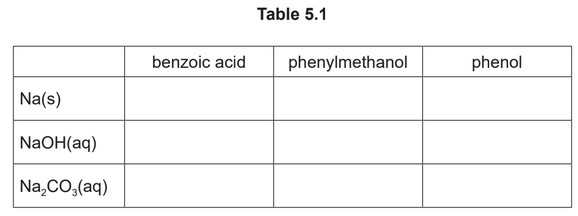
(c) (i) Benzoyl chloride, \(C_6H_5COCl\), can be synthesised by the reaction of benzoic acid with either \(PCl_5\) or \(SOCl_2\).
Complete the equations for these reactions.
reaction 1 \(C_6H_5COOH + PCl_5 → C_6H_5COCl\) + …………………… + ……………………
reaction 2 \(C_6H_5COOH + SOCl_2 → C_6H_5COCl\) + …………………… + ……………………
(ii) Use your answer to (c)(i) to suggest why it is easier to isolate, in a pure form, the \(C_6H_5COCl\) from reaction 2 compared to reaction 1.
(d) Benzoyl chloride is hydrolysed by water at room temperature to form benzoic acid.
(i) Complete the diagram to show the mechanism for the reaction between \(C_6H_5COCl\) and \(H_2O\).
Include charges, dipoles, lone pairs of electrons and curly arrows as appropriate.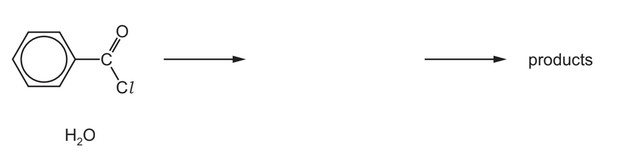
(ii) Name the type of mechanism you showed in (d)(i).
(e) Acyl chlorides react with sodium carboxylates to form acid anhydrides as shown in Fig. 5.1.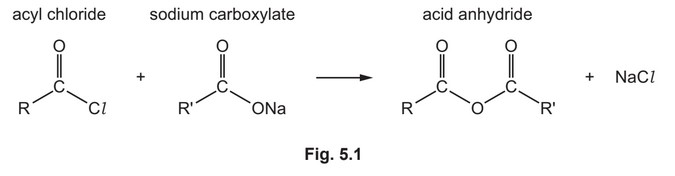
The condensation polymers, polyanhydride and polyester, are formed by similar methods.
The repeat unit for a polyanhydride is shown in Fig. 5.2.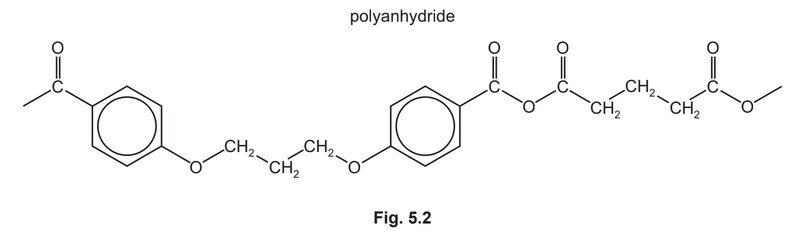
(i) Use Fig. 5.1 and Fig. 5.2 to suggest the structures of the two monomers used to make this polyanhydride.
(ii) Polyanhydrides are biodegradable polymers.
Suggest how this polyanhydride can be degraded.
Answer/Explanation
Answer:
(a) M1 benzoic acid > phenol > phenylmethanol
M2 / M3 Any two of:
in benzoic acid negative inductive effect of C=O AND O-H bond is weakened
OR due to delocalisation of minus charge by C=O / 2O carboxylate ion is stabilised
in phenol lone pair on oxygen is delocalised into the ring AND O-H bond is weakened
in phenyl methanol positive inductive effect of \(CH_2\) group AND O-H bond is strengthened
(b) 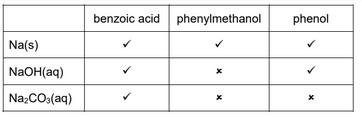
Three correct for one mark, six correct for two marks, nine correct for three marks
(c) (i) \(POCl_3\) and HCl AND \(SO_2\) and HCl
(ii) all the by-products / SO2 and HCl are gaseous OR no liquid by-products formed
(d) (i) 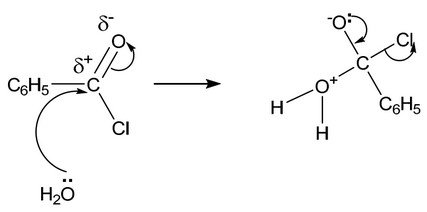
On the left-hand side:
lone pair on O
correct arrow from O to C (of C=O)
dipole on C=O
correct arrow on C=O
M1 / M2 Two correct for one mark, four correct for two marks
On the right-hand side:
M3 correct intermediate
M4 arrow from lone pair on O– to C-O bond AND arrow from C-Cl to Cl
(ii) addition-elimination
(e) (i) 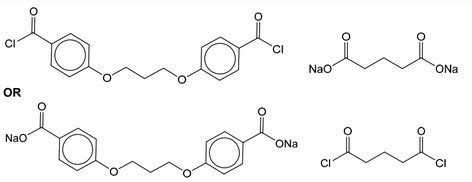
(ii) hydrolysis OR heating in dilute acid / alkali