Question
(a) Compare and explain the relative acidities of butanoic acid, ethanol, ethanoic acid and water.
(b) Three carboxylic acids, methanoic acid, HCO2H, ethanedioic acid, HO2CCO2H, and butanedioic acid, HO2CCH2CH2CO2H, are compared. Two tests were carried out on separate samples of each organic acid, as shown.
The following results were obtained. ✔= observed change 🗙 = no observed reaction
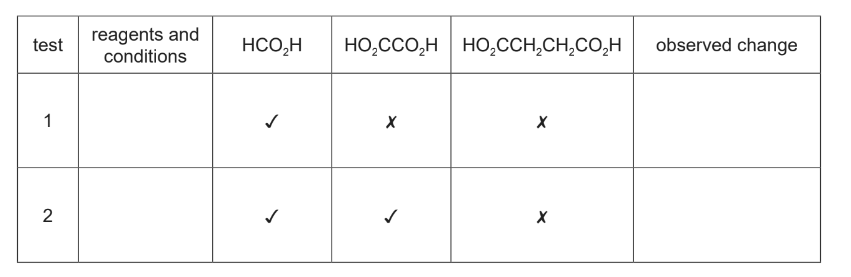 (i) Complete the table with the reagents and conditions and the observed change for a positive test. Assume these organic acids all have a similar acid strength.
(i) Complete the table with the reagents and conditions and the observed change for a positive test. Assume these organic acids all have a similar acid strength.
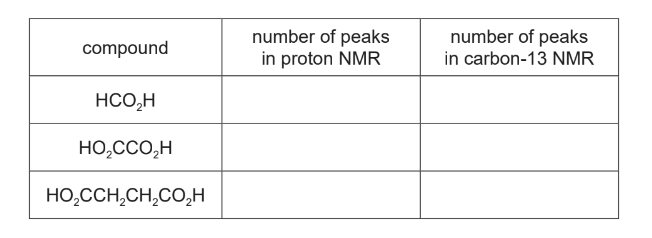
(ii) Each compound, HCO2H, HO2CCO2H and HO2CCH2CH2CO2H, is dissolved seperately in CDCl3. Proton (¹H) NMR and carbon-13 (¹³C) NMR spectra are then obtained.Complete the table.
(iii) The proton NMR spectrum of HCO2H in D2O is obtained.
Describe and explain the difference observed between this spectrum and the proton NMR spectrum of HCO2H in (b)(ii).
(c) 1,4-dibromobutane, Br(CH2)4Br, is used in the synthesis of the dicarboxylic acid J and diamine K as shown.
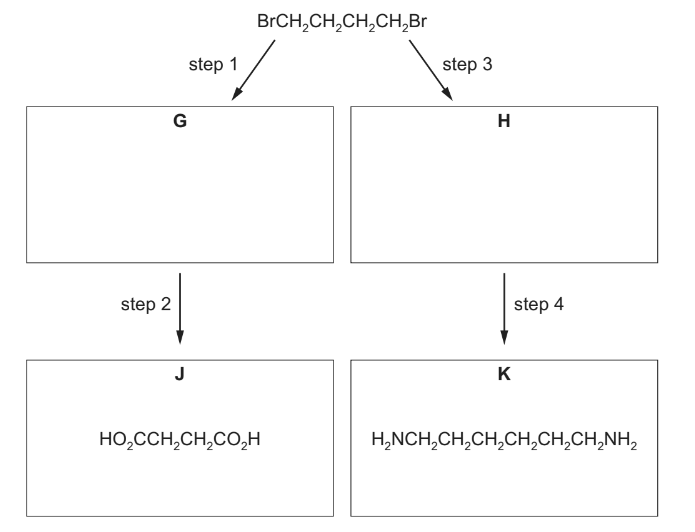
(i) Draw the structures of G and H in the boxes.
(ii) Suggest reagents and conditions for each of steps 1 to 4.
(d) Polyamide L can be synthesised from dicarboxylic acid J, HO2C(CH2)2CO2H, and diamine K, H2N(CH2)6NH2.
Draw the repeat unit of the polymer formed in the box. Any functional groups should be shown displayed.
Answer/Explanation
Answer: (a) M1: ethanoic acid > butanoic acid > water > ethanol
M2: a reason given in terms of an electron donating or an electron withdrawing group for one of:
strengthening of O–H bond OR weakening of O–H bond OR stability of anion
Two out of the three alternatives M3, M4 and M5:
M3: ethanol: positive inductive effect / electron donating effect of ethyl / alkyl / R group
M4: butanoic acid: positive inductive effect / electron donating effect of propyl / alkyl / R group
M5: (either ethanoic or butanoic) acid: negative inductive effect of either C=O or carbonyl OR negative charge delocalised
over COO–
(b)(i)
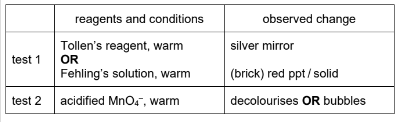
M1 / M2: reagents and conditions × 2
M3: observations both correct
(b)(ii)
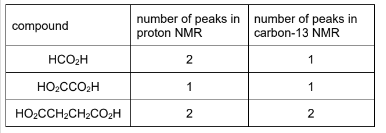
one mark for three, four or five correct
two marks for six correct
(b)(iii) OH peak disappears AND proton / H exchanges with deuterium 1
(c)(i) G = HOCH2CH2CH2CH2OH
H = NCCH2CH2CH2CH2CN
(c)(ii) M1: step 1 NaOH(aq) + heat
M2: step 2 acidified KMnO4 + heat / acidified K2Cr2O7 + heat
M3: step 3 CN– / KCN / NaCN + heat
M4: step 4 LiAlH4 ALLOW Na in ethanol or H2 + Ni / Pd / Pt
(d) 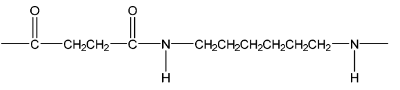
M1: correct displayed amide linkage
M2: the rest of the repeat unit correct including trailing bonds
Question
CH3CH2Cl CH3COCl C6H5Cl
(b) Epibatidine is a naturally occurring organochlorine compound.
(c) Polyamides, such as nylon-6, can be prepared from a monomer that contains both an amine and an acyl chloride functional group.
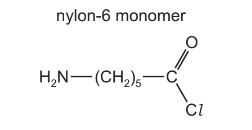
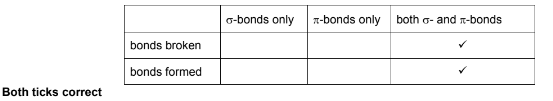
(i) When the nylon-6 monomer is hydrolysed, bonds are broken and formed.
By considering the two steps in the mechanism of the reaction, complete the table by placing one tick (✔) in each row to indicate the types of bonds broken and formed during the mechanism.
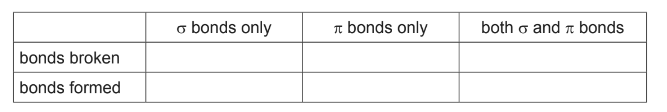
(ii) Draw two repeat units of nylon-6. The amide bond should be shown fully displayed.
(d) An addition polymer made from two different alkene monomers is called a co-polymer. A section of a polyalkene co-polymer is shown.
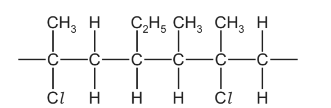
Draw the structure of the two alkene monomers which produce this co-polymer.
(e) Explain why polyamides normally biodegrade more readily than polyalkenes.
(f) The alkene phenylethene can be prepared from benzene in three steps.
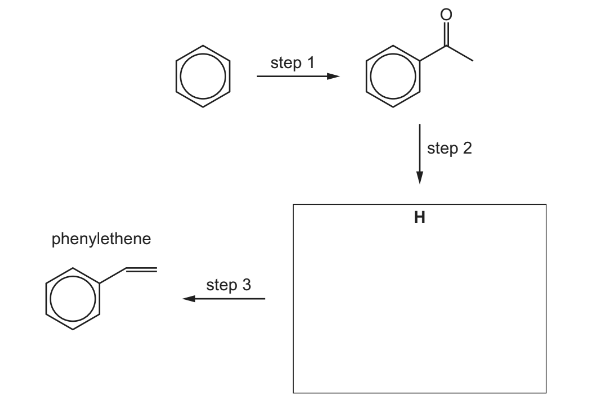
(i) Deduce the identity of compound H and draw its structure in the box.
(ii) Suggest reagents and conditions for each of the steps 1–3.
Answer/Explanation
Answer: (a) M1: CH3COCl > CH3CH2Cl > C6H5Cl
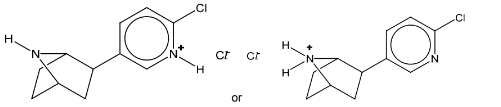
(c)(ii)
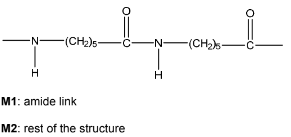
d)
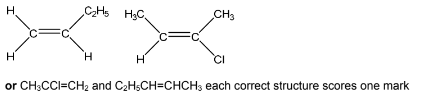
(e) C-C bonds are non-polar / polyalkenes cannot be hydrolysed and polyamides can be broken down by hydrolysis
(f)(i)
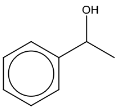
(f)(ii) M1: step 1: CH3COCl + AlCl3
M2: step 2: NaBH4 / LiAlH4
M3: step 3: conc. H2SO4, heat